Great collaboration with Danette Daniels’s group at Promega!
Read the Open Access full article.
Read the UoD News article: “Three heads are better than two in drug discovery concept“
Authors: Satomi Imaide, Kristin M. Riching, Nikolai Makukhin, Vesna Vetma, Claire Whitworth, Scott J. Hughes, Nicole Trainor, Sarah D. Mahan, Nancy Murphy, Angus D. Cowan, Kwok-Ho Chan, Conner Craigon, Andrea Testa, Chiara Maniaci, Marjeta Urh, Danette L. Daniels* & Alessio Ciulli*
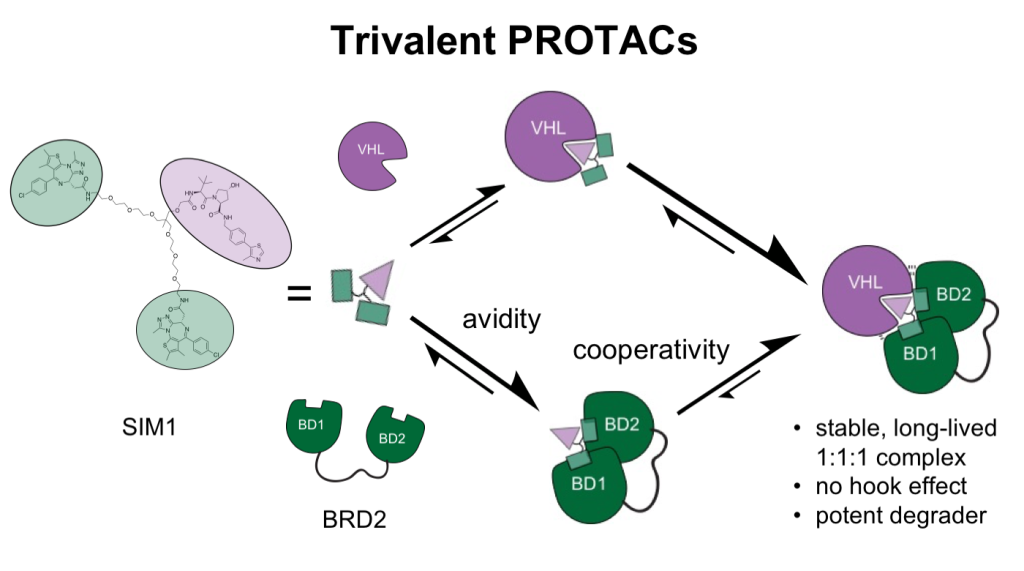
Title: Trivalent PROTACs enhance protein degradation via combined avidity and cooperativity
Abstract
Bivalent proteolysis-targeting chimeras (PROTACs) drive protein degradation by simultaneously binding a target protein and an E3 ligase and forming a productive ternary complex. We hypothesized that increasing binding valency within a PROTAC could enhance degradation. Here, we designed trivalent PROTACs consisting of a bivalent bromo and extra terminal (BET) inhibitor and an E3 ligand tethered via a branched linker. We identified von Hippel–Lindau (VHL)-based SIM1 as a low picomolar BET degrader with preference for bromodomain containing 2 (BRD2). Compared to bivalent PROTACs, SIM1 showed more sustained and higher degradation efficacy, which led to more potent anticancer activity. Mechanistically, SIM1 simultaneously engages with high avidity both BET bromodomains in a cis intramolecular fashion and forms a 1:1:1 ternary complex with VHL, exhibiting positive cooperativity and high cellular stability with prolonged residence time. Collectively, our data along with favorable in vivo pharmacokinetics demonstrate that augmenting the binding valency of proximity-induced modalities can be an enabling strategy for advancing functional outcomes.
Our PROTAC degrader SIM1 is available from Tocris, together with its negative control cis-SIM1
