We are happy to share our new publication on the structure-guided improvement of our von Hippel–Lindau (VHL) inhibitors | Just out ASAP in the Journal of Medicinal Chemistry
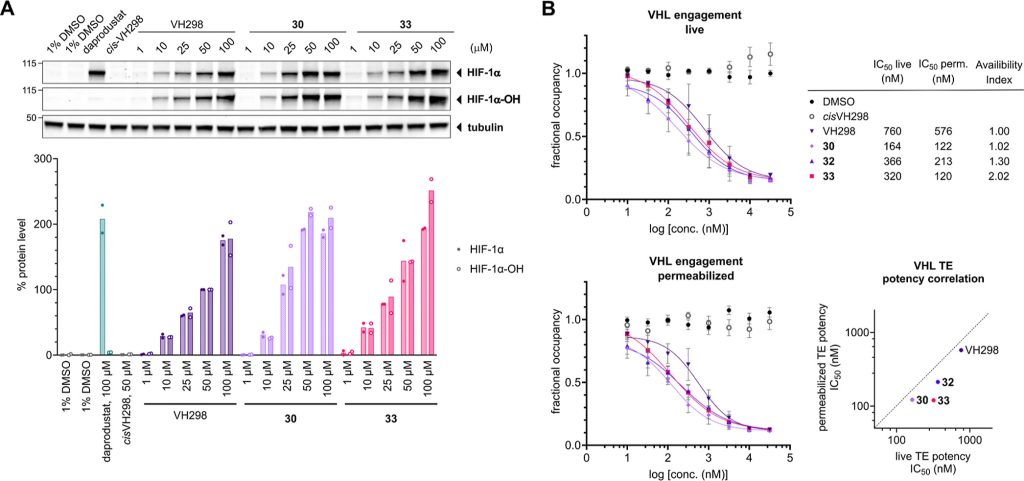
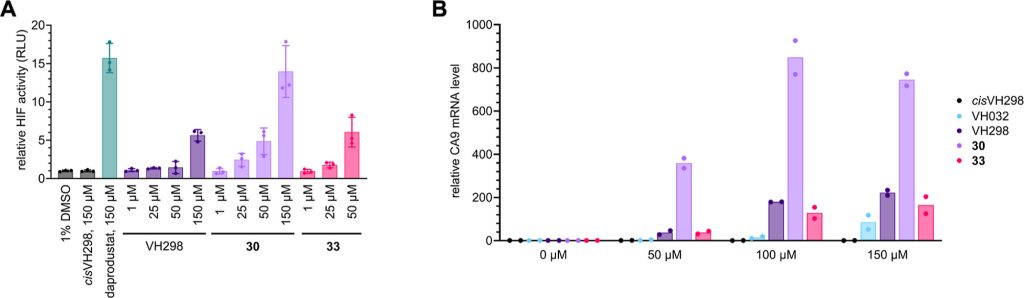
We design a new di-Methyl analogue of VH101 (cmpnd 30) and qualify it as the most potent VHL inhibitor in vitro and in cell. This work has been a fantastic collaboration with Michael Gütschow group and colleagues at the University of Bonn. The work work spearheaded by Lan who visited our group twice to conduct key experiments for this paper, and has involved highly producted team-work collaboration with Claudia, Ryan and Adam in the group who desgined and performed the cell biology, crystallography and biophysical binding experiments, respectively. Together they brilliantly championed collaboration, to qualify the improved VHL inhibitors – first biophysically and structurally for binding properties and binding modes, and then in cell-based assays for target engagement and HIF-1alpha stabilization and activity. And big thanks also to the Bonn and Ljubjana teams, including Christian Steinebach, Izidor Sosic, Alesa Bricelj and their coworkers for their amazing contributions to the enabling chemistry effort.
We look forward to seeing how the field leverages these new designs and findings, both as new hypoxia inducing probes, and as VHL ligand in PROTACs. We are already finding these v useful in our own degrader discovery programmes!,
Read the full article Open Access
Authors: Lan Phuong Vu, Claudia J. Diehl, Ryan Casement, Adam G. Bond, Christian Steinebach, Nika Strasěk, Alesǎ Bricelj, Andrej Perdih, Gregor Schnakenburg, Izidor Sosic,̌ Alessio Ciulli,* and Michael Gütschow*
Title: Expanding the Structural Diversity at the Phenylene Core of Ligands for the von Hippel–Lindau E3 Ubiquitin Ligase: Development of Highly Potent Hypoxia-Inducible Factor-1α Stabilizers
Abstract
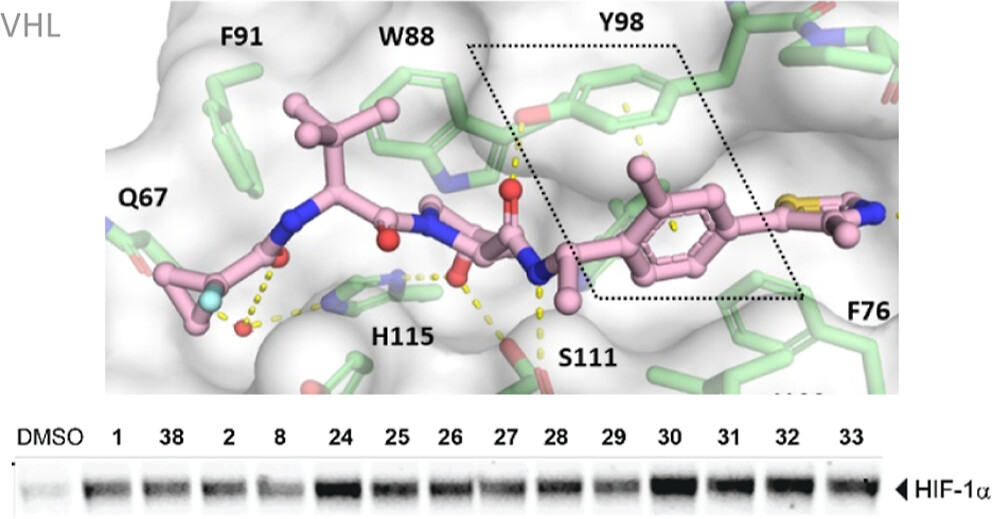
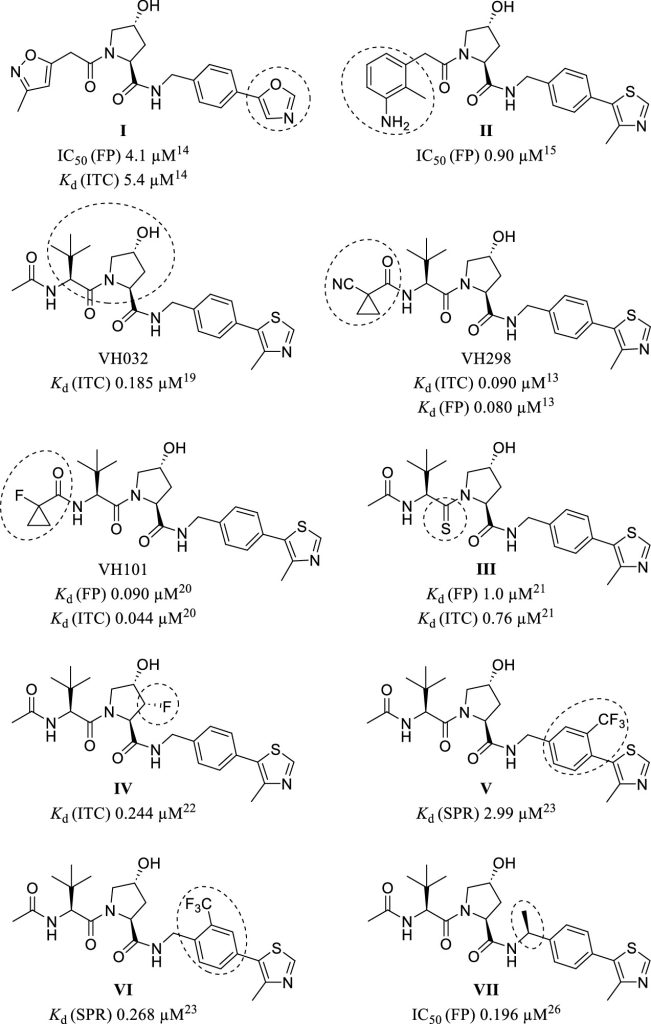
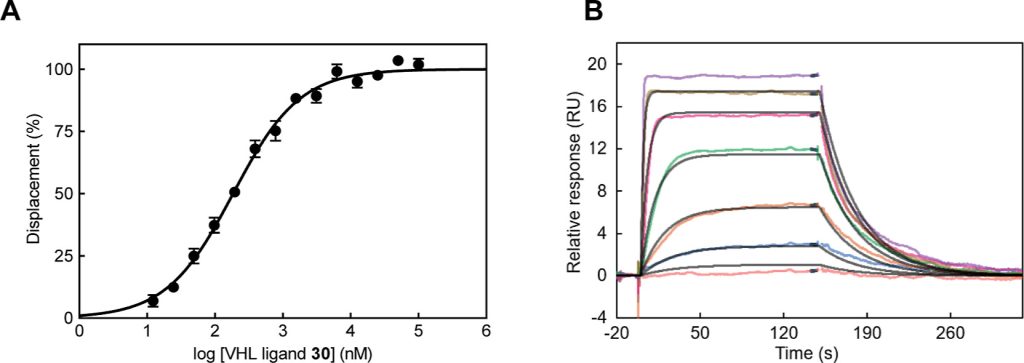
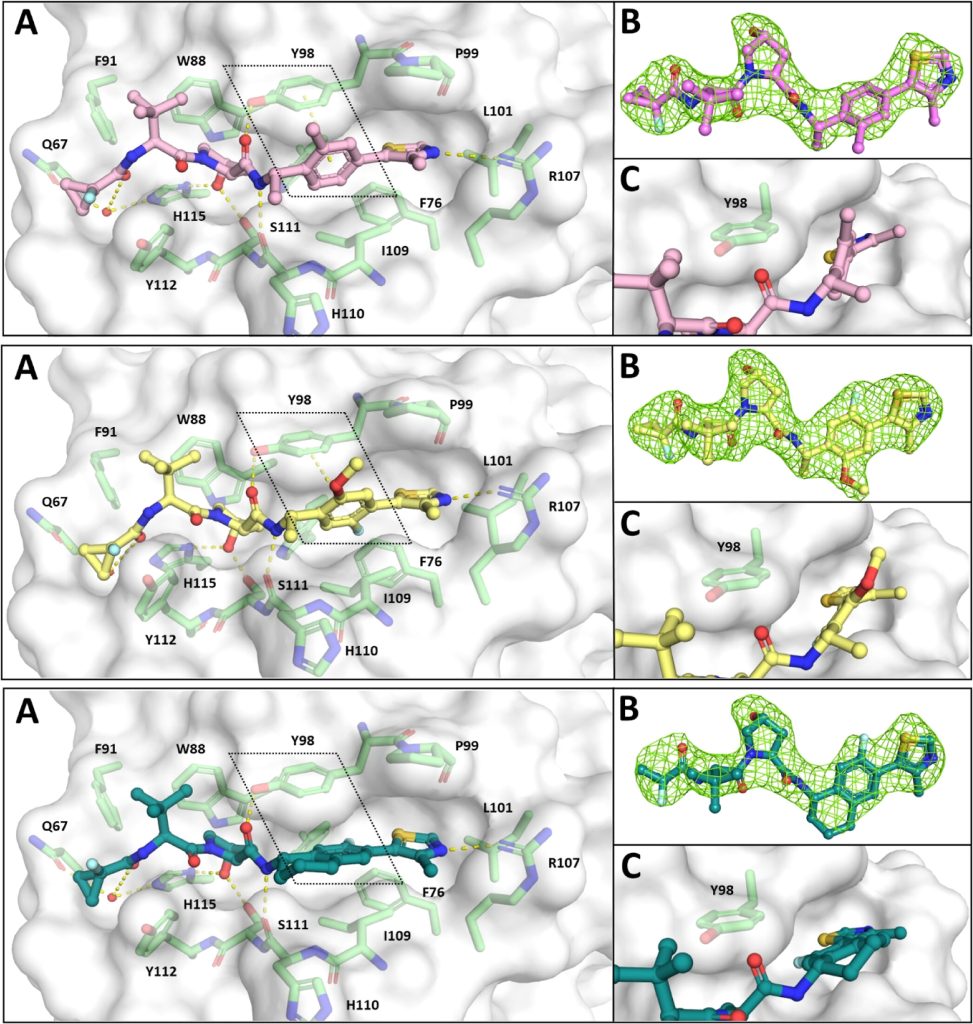
Hypoxia-inducible factor-1α (HIF-1α) constitutes the principal mediator of cellular adaptation to hypoxia in humans. The HIF-1α protein level and activity are tightly regulated by the ubiquitin E3 ligase von Hippel–Lindau (VHL). Here, we performed a structure-guided and bioactivity-driven design of new VHL inhibitors. Our iterative and combinatorial strategy focused on chemical variability at the phenylene unit and encompassed further points of diversity. The exploitation of tailored phenylene fragments and the stereoselective installation of the benzylic methyl group provided potent VHL ligands. Three high-resolution structures of VHL–ligand complexes were determined, and bioactive conformations of these ligands were explored. The most potent inhibitor (30) exhibited dissociation constants lower than 40 nM, independently determined by fluorescence polarization and surface plasmon resonance and an enhanced cellular potency, as evidenced by its superior ability to induce HIF-1α transcriptional activity. Our work is anticipated to inspire future efforts toward HIF-1α stabilizers and new ligands for proteolysis-targeting chimera (PROTAC) degraders.