Amide-to-ester substitution as a strategy for optimizing PROTAC permeability and cellular activity. Great work by Adam, Conner, and Tori Klein from Scott Lokey’s lab.
Authors: Klein, V.G., Bond, A.G, Craigon, C., Lokey, R.S., Ciulli, A.*
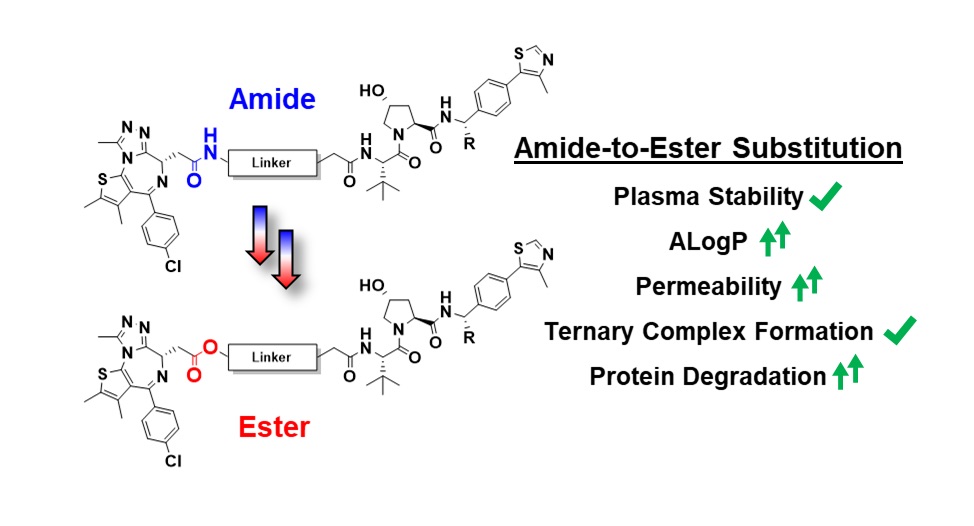
Title: Amide-to-ester substitution as a strategy for optimizing PROTAC permeability and cellular activity
Abstract
Bifunctional PROTAC degraders belong to “beyond Rule of 5” chemical space, and criteria for predicting their drug-like properties are underdeveloped. PROTAC components are often combined via late-stage amide couplings, due to the reliability and robustness of amide bond formation. Amides, however, can give rise to low cellular permeability and poor ADME properties. We hypothesized that a bioisosteric replacement of an amide with a less polar ester could lead to improvements in both physicochemical properties and bioactivity. Using a library of model compounds, bearing either amides or esters at various linker-warhead junctions, we identify parameters for optimal compound lipophilicity and permeability. We next applied these learnings to design a set of novel amide-to-ester substituted, VHL-based BET degraders with increased permeability. Our ester-PROTACs remarkably retained intracellular stability, were overall more potent degraders than their amide counterparts and showed an earlier onset of the hook effect. These enhanced cellular features were found to be driven by greater cell permeability rather than improvements in ternary complex formation. This largely unexplored amide-to-ester substitution therefore provides a simple and practical strategy to enhance PROTAC permeability and degradation performance. Such approach could prove equally beneficial to other classes of beyond Ro5 molecules.
