Check our latest paper published in Angewandte.
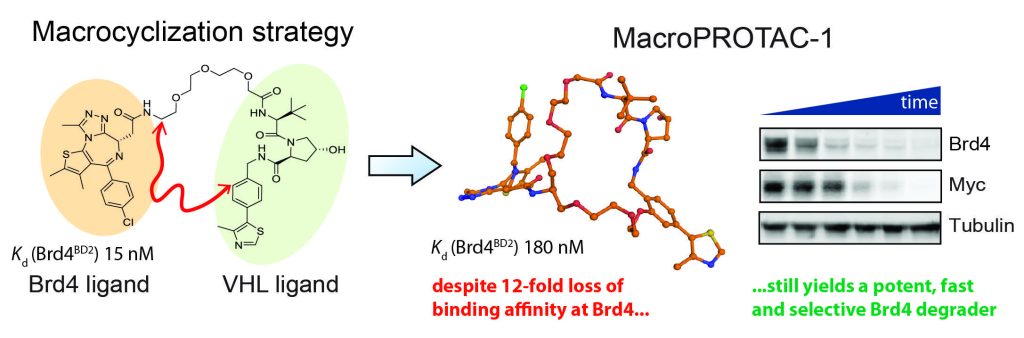
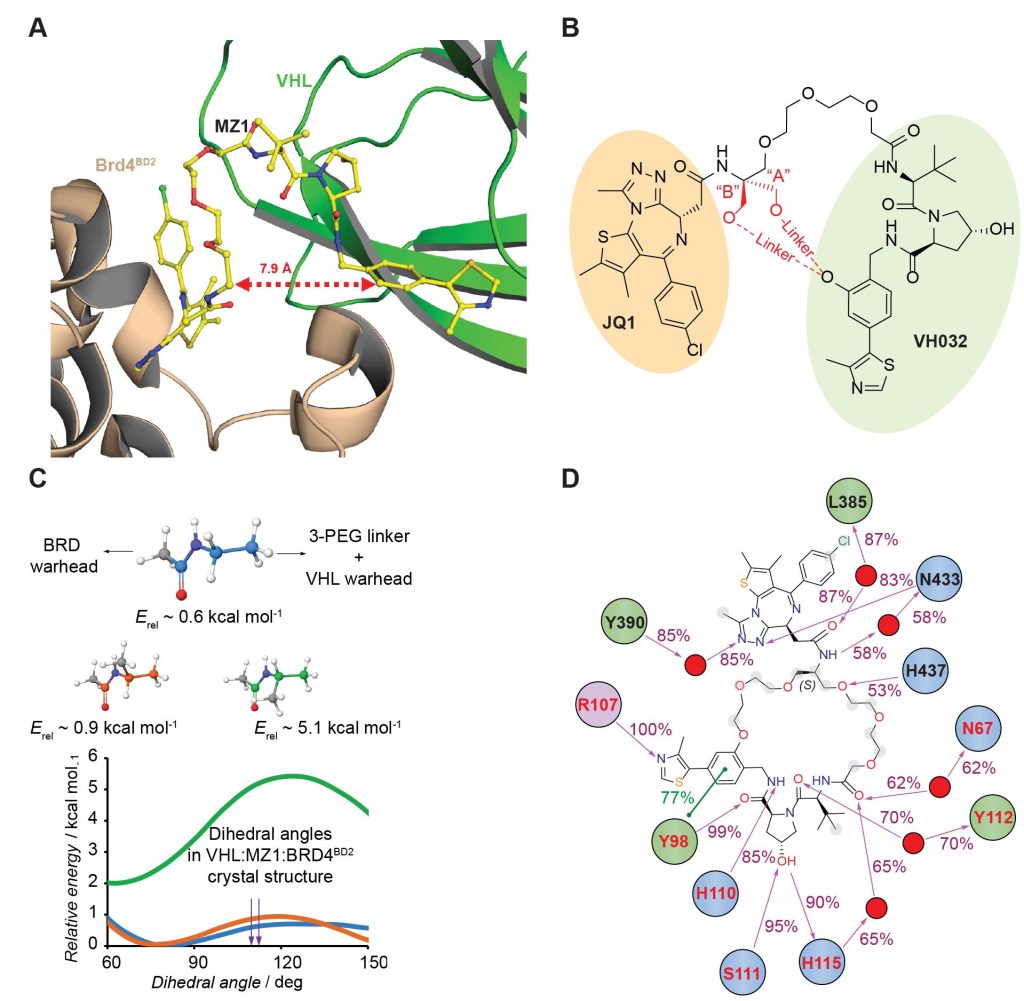
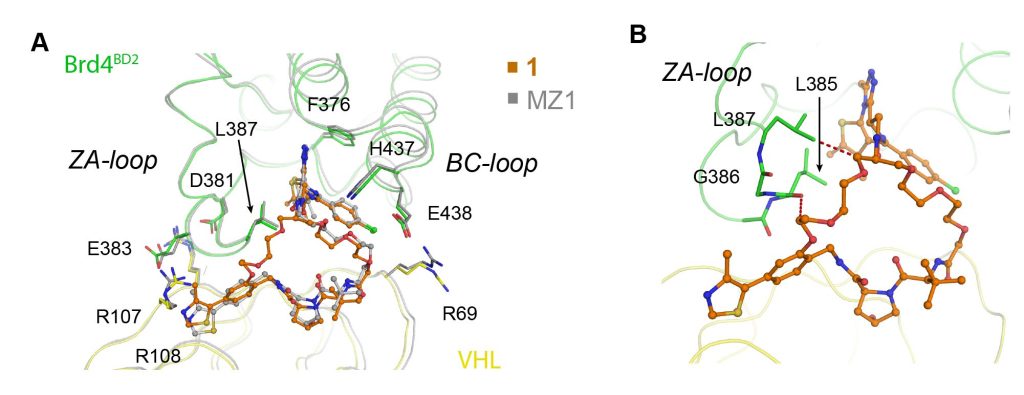
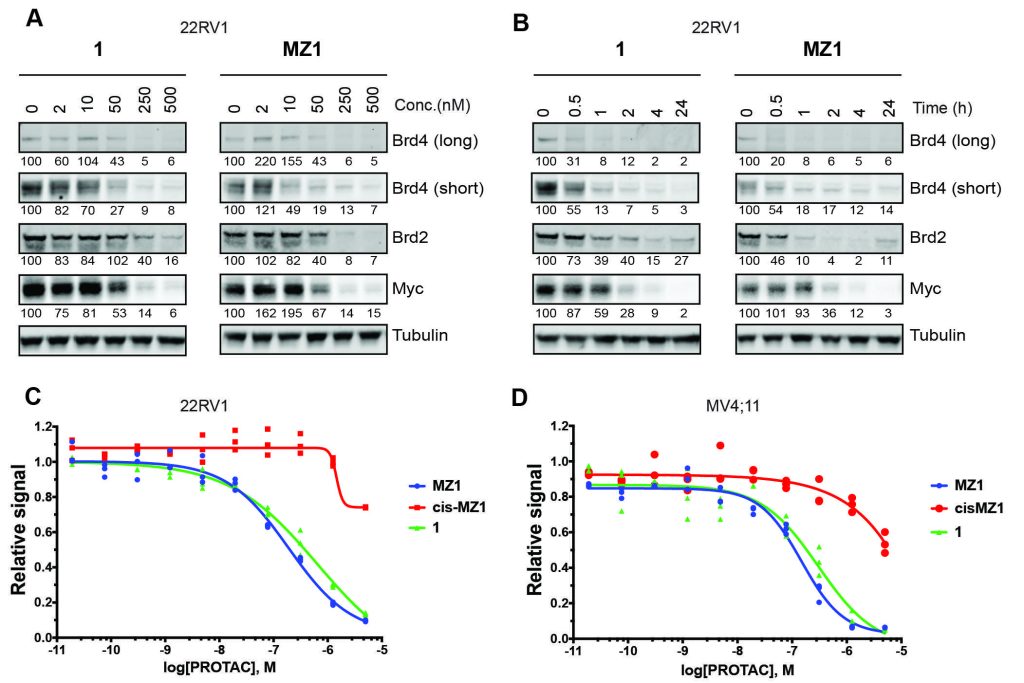
Closing the circle: A macrocyclic PROTAC has been designed and synthesized by adding a cyclizing linker to MZ1, a PROTAC targeting the bromodomain of the BET protein, Brd4. A co‐crystal structure of macroPROTAC‐1 bound in a ternary complex with E3 ligase VHL and the second bromodomain of Brd4 validated the rational design. This macrocyclization strategy enhanced the target specificity and cellular activity of the PROTAC.
Read University of Dundee press release: “PROTACs go macrocyclic“
Title: Structure‐Based Design of a Macrocyclic PROTAC
Authors: Dr. Andrea Testa Dr. Scott J. Hughes Dr. Xavier Lucas Dr. Jane E. Wright Prof. Dr. Alessio Ciulli*
Abstract
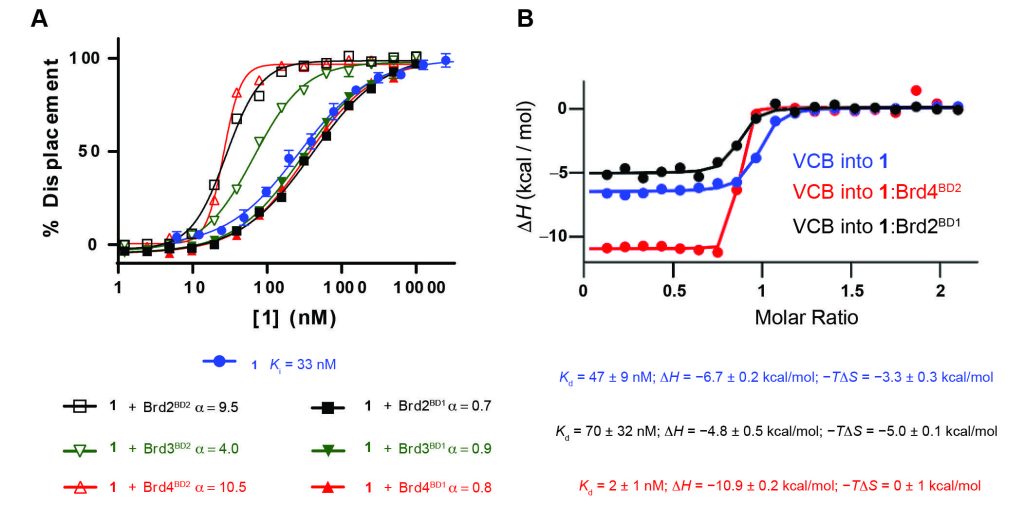
Constraining a molecule in its bioactive conformation via macrocyclization represents an attractive strategy to rationally design functional chemical probes. While this approach has been applied to enzyme inhibitors or receptor antagonists, to date it remains unprecedented for bifunctional molecules that bring proteins together, such as PROTAC degraders. Herein, we report the design and synthesis of a macrocyclic PROTAC by adding a cyclizing linker to the BET degrader MZ1. A co‐crystal structure of macroPROTAC‐1 bound in a ternary complex with VHL and the second bromodomain of Brd4 validated the rational design. Biophysical studies revealed enhanced discrimination between the second and the first bromodomains of BET proteins. Despite a 12‐fold loss of binary binding affinity for Brd4, macroPROTAC‐1 exhibited cellular activity comparable to MZ1. Our findings support macrocyclization as an advantageous strategy to enhance PROTAC degradation potency and selectivity between homologous targets.