‘Trojan Horse’ chemical tool allows researchers to target ‘undruggable’ proteins
University of Dundee researchers have developed a new molecule that is able to target and bind to a protein class previously considered undruggable by using a masking technique to enter the cell.
A team in the laboratory of Professor Alessio Ciulli, at the University’s new Centre for Targeted Protein Degradation (CeTPD), have unlocked a class of proteins known as suppressor of cytokine signalling (SOCS). SOCS proteins contain a crucial structural portion called the SH2 domain which, until now, was thought of as undruggable.
The SOCS2 protein is involved in many diseases, including cancer and inflammatory diseases, and until now, no small molecule has ever been generated that successfully inhibits the protein.
One of the major research interests of the Ciulli Lab is the targeting of E3 ligase complexes, and SOCS2 is part of one such complexes. These complexes initiate the recycling of proteins in the cell, by earmarking abnormal proteins for destruction.
It is possible to induce this process using carefully designed drugs, known as PROTACs, however this requires molecules that tightly bind a certain E3 ligase – SOCS2 is an example of one such ligase. The research team have designed a molecule that does exactly that, offering a chemical tool able to study the function of these proteins and manipulate them in ways not previously possible.
The research, funded by the European Research Council (ERC) and the Innovative Medicine Initiative 2 consortium EUbOpen, is published in the journal Nature Communications.
“There is growing interest in targeting SH2 domains using small molecules because they are so prevalent and involved in so many interactions of so many proteins that determine key cellular processes,” explained Professor Ciulli, Director of the CeTPD.
“When these processes go awry in disease, by targeting these interactions we have a very effective way to block the progression of the disease, and even revert it. Beyond this, in separate approaches we can co-opt or hijack key signalling proteins as in case of our targeted SOCS2 in order to re-direct their activity against disease-causing proteins, in a therapeutically beneficial manner. To enable either of these strategies, a critical first step involves finding small molecules that provide suitable starting points. With this work, we have done just that, for a protein class that had to date proven recalcitrant to these types of approaches.”
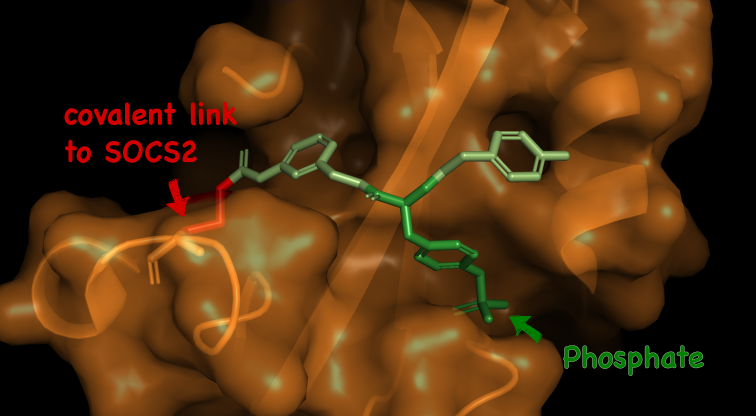
SOCS2 and its SH2 domain have very shallow pockets which are difficult to effectively target with molecules that have the properties required to become drugs. The natural binder of the SH2 domain contains a phosphate group, which is highly negatively charged.
To develop the molecule, the Dundee team worked to evolve around the SH2 domain pocket, designing a compound that could engage with the protein binding site. However, the compound developed by the Dundee researchers also contains a phosphate unit, causing significant problems for getting it to enter the cell. By ‘masking’ the charged group, the researchers were able to successfully target the protein.
“The charged group is repelled by the negatively charge surface of the cell, and so it keeps the drug on its exterior. One strategy to overcome this is to mask the charged group in a sort of Trojan Horse allegory.” explained Dr Sarath Ramachandran, who was a postdoctoral researcher in the Ciulli Lab at the time of the work.
“Groups that mask the charge are incorporated, which allows the drug to enter the cell. Once inside, a spontaneous chemical process mediated by the environment inside the cell happens, which tears apart such groups to ‘unmask’ the drug. This is akin to the Greeks jumping out of the horse inside which they were hiding. This releases the ‘active’ drug, allowing it to engage our target protein – much akin to the Greeks now being free to attack the city of Troy.
“Then, by permanently latching onto the protein we can block the target and prevent it from recruiting its native interacting proteins, providing a new research tool that will be useful to study the biological role and downstream signalling of SOCS2. This opens an opportunity to develop new therapeutics that target this protein and allows us to study and further and target the protein in ways not previously possible.”
Professor Ciulli added, “In the short-term, other medicinal chemists and cell biologists will benefit from our new molecule, which can be used to study the SOCS2 protein in detail – namely its cellular function and downstream effects. On a larger timescale, our compound can be further elaborated into potential new drugs, for the treatment of cancer and other diseases.”
The work was spearheaded by postdoctoral researchers Dr Sarath Ramachandran and Dr Nikolai Makukhin, working as a multi-disciplinary team with other researchers in the Ciulli Lab including Dr Kevin Haubrich, Dr Dylan Lynch and MRes student Beth Forrester, as shown in the feature image above.