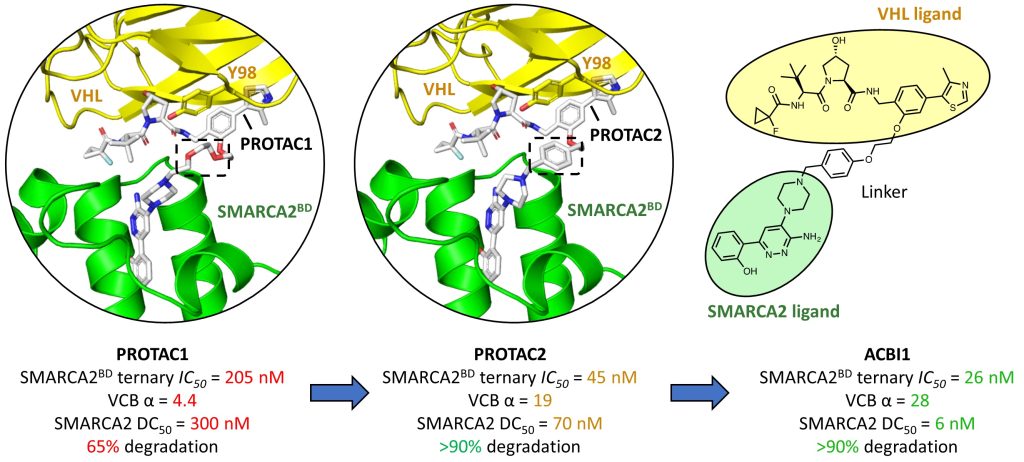
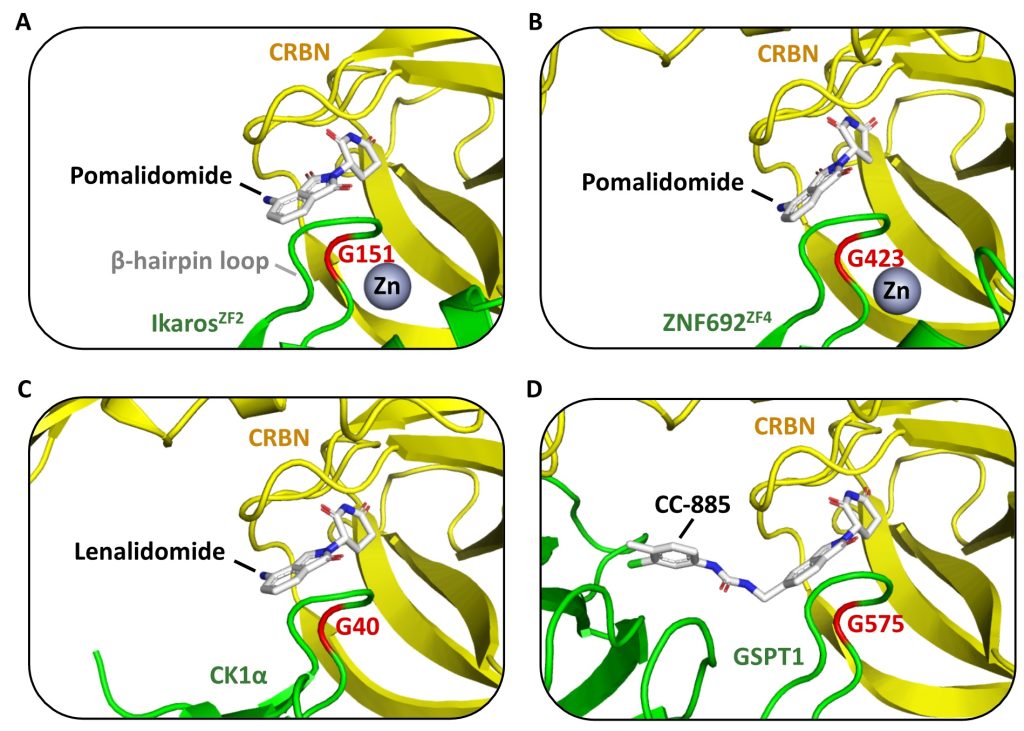
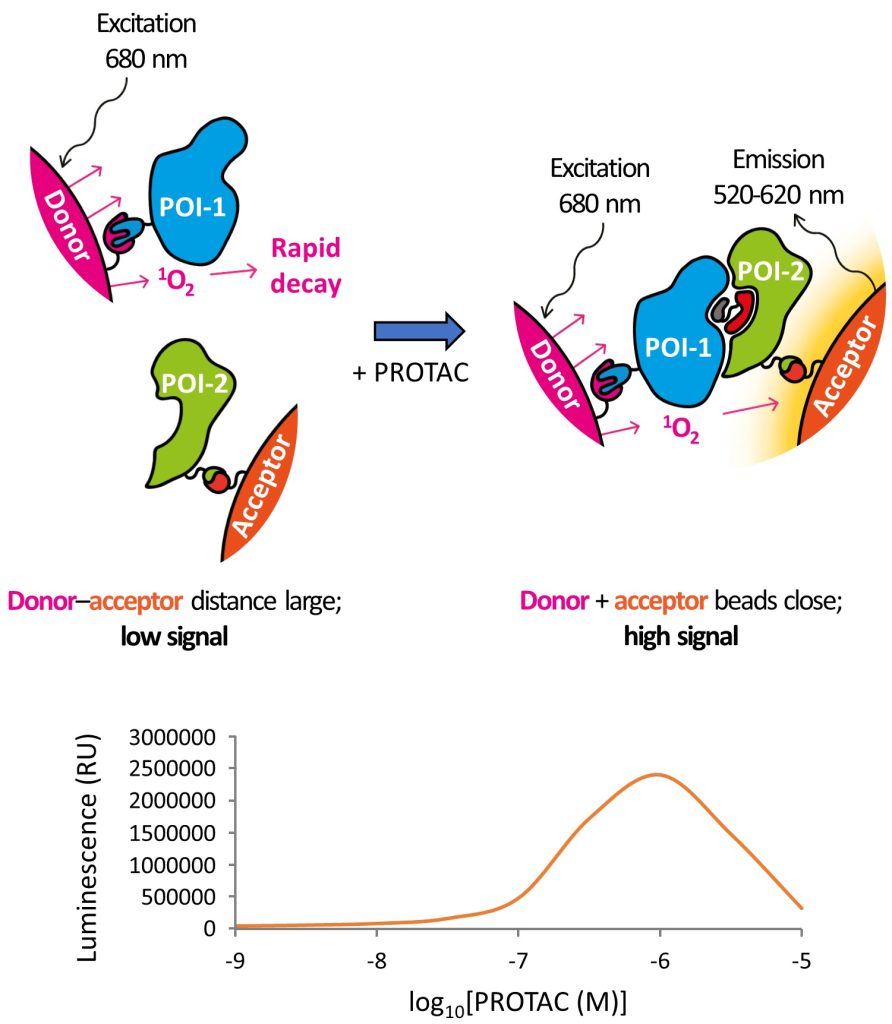
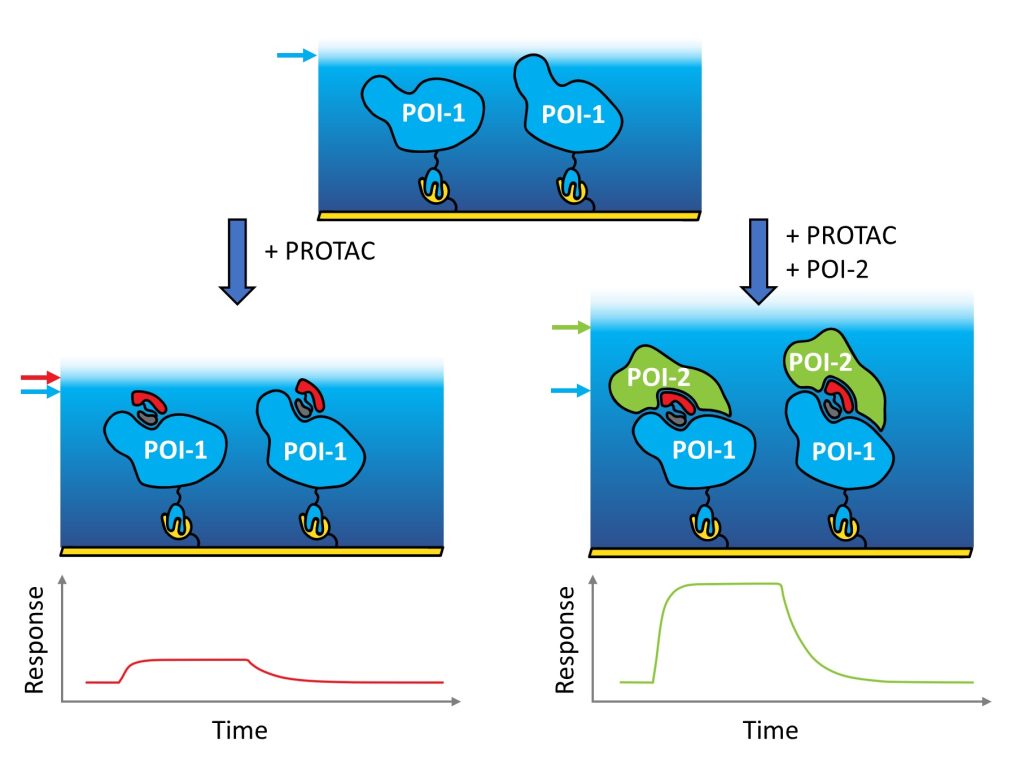
Well done to David – great job on this chapter!
Authors: David Zollman, Alessio Ciulli*
Title: Structural and Biophysical Principles of Degrader Ternary Complexes
Abstract
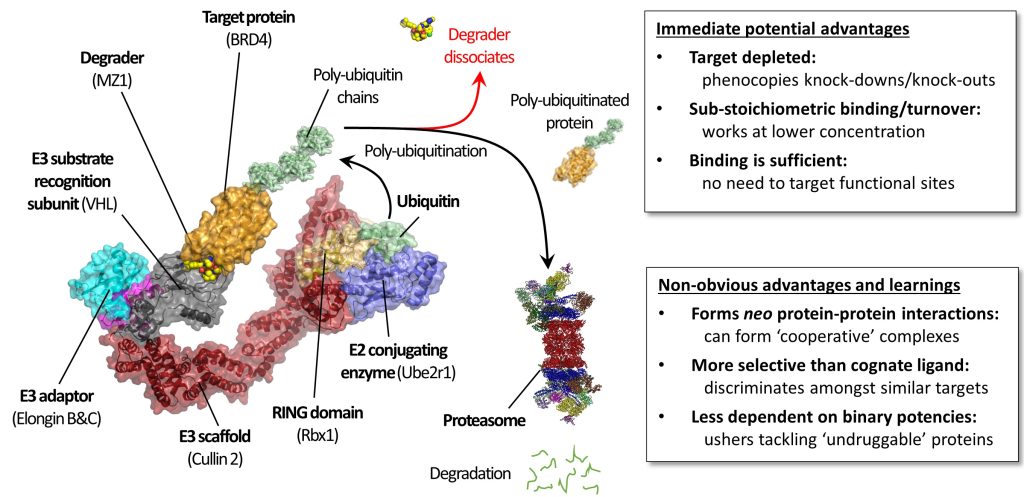
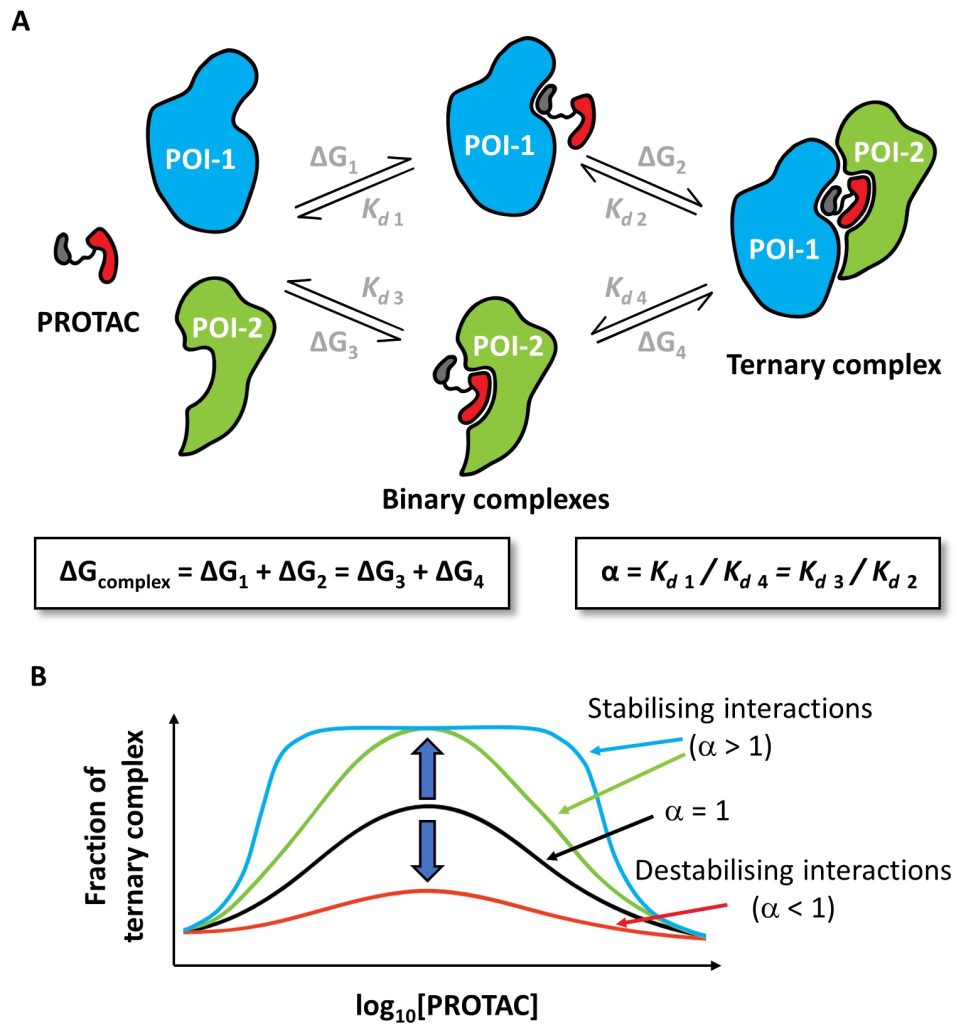
Small-molecule degraders are a revolutionary modality of pharmacological intervention in chemical biology and drug discovery. Instead of inhibiting protein targets, molecules that induce rapid, profound and selective degradation of targeted proteins are being developed as biological tools and investigational therapeutics. Degraders predominantly recruit a target protein to an E3 ubiquitin ligase and so form with them a ternary complex, which triggers target ubiquitination and subsequent proteasomal degradation. The structural, thermodynamic and kinetic features of the ternary complexes underpin degraders’ mode of action and determine the speed, potency, selectivity and durability of their cellular degradation activity.
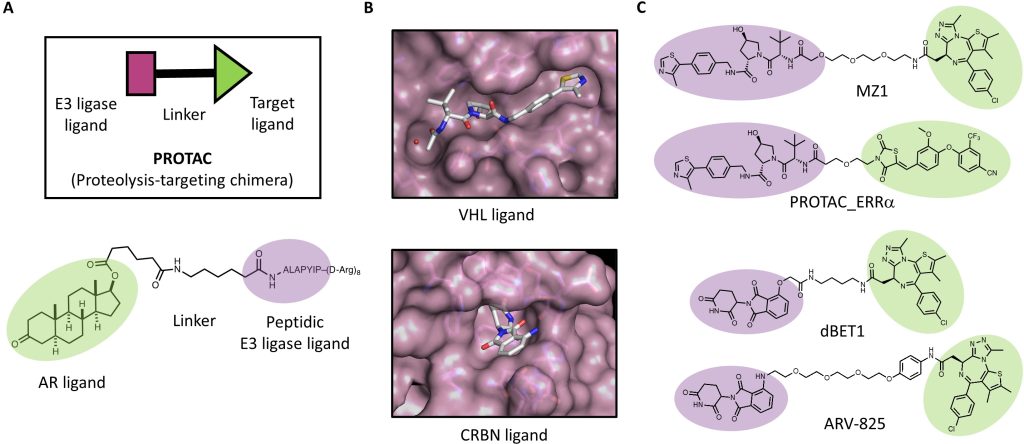
In this chapter, we briefly recount the history of how degrader molecules have come to the fore, with a particular focus on bifunctional degrader molecules, popularly known as proteolysis-targeting chimeras (PROTACs).
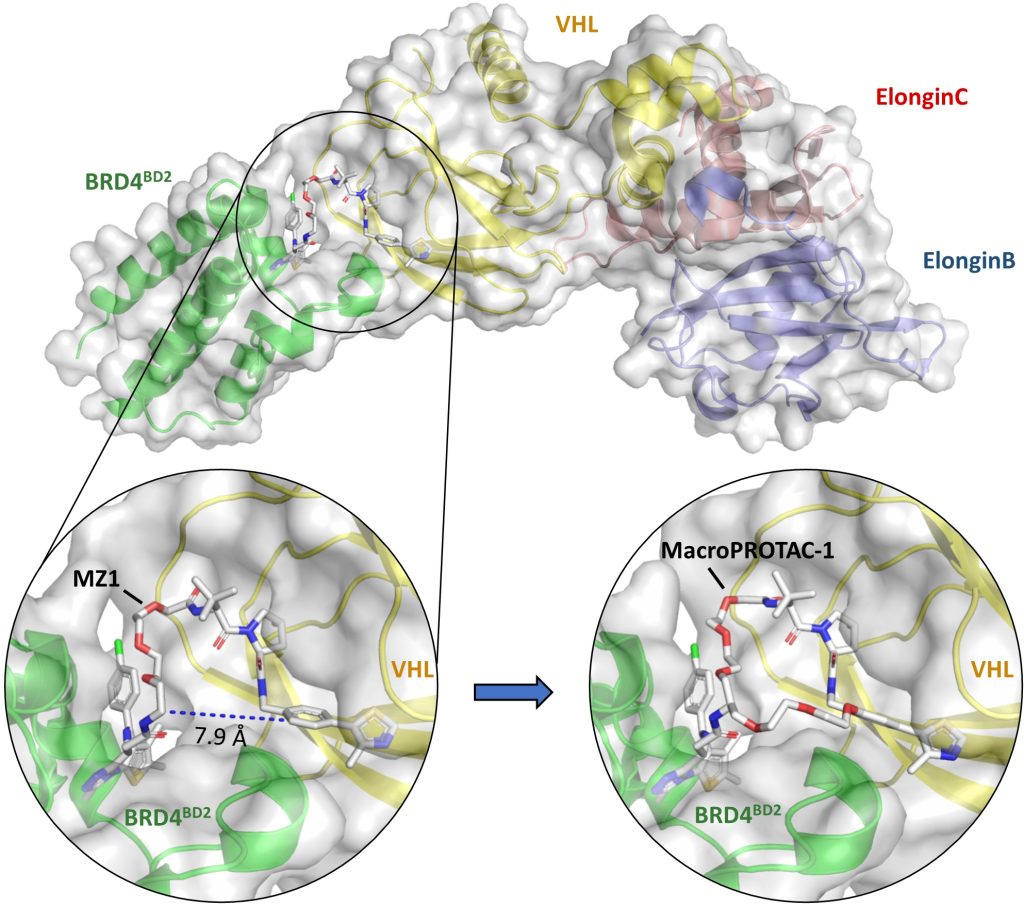
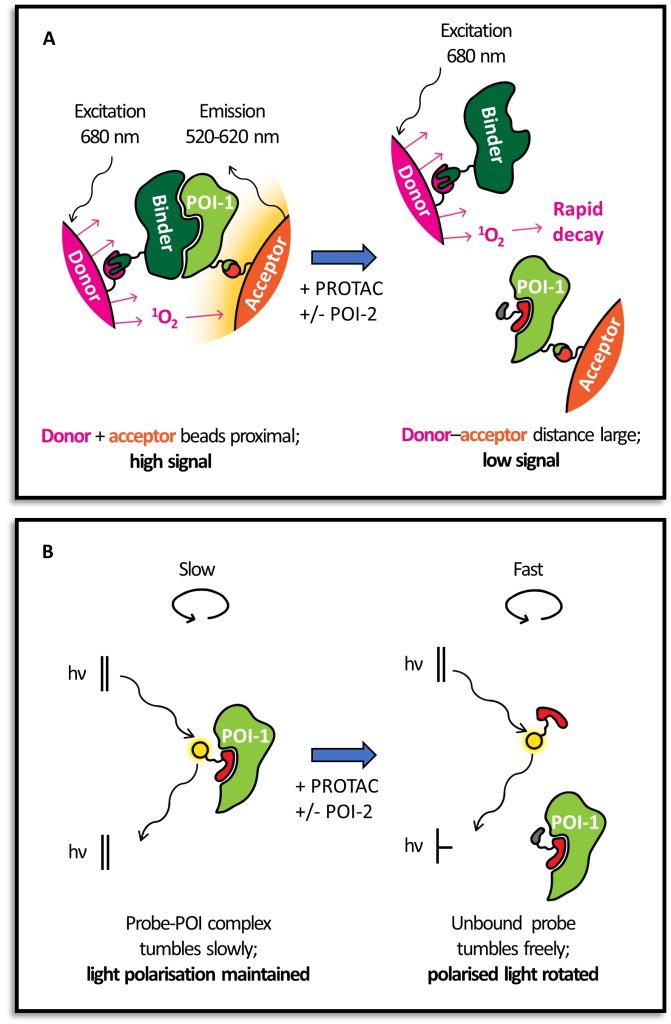
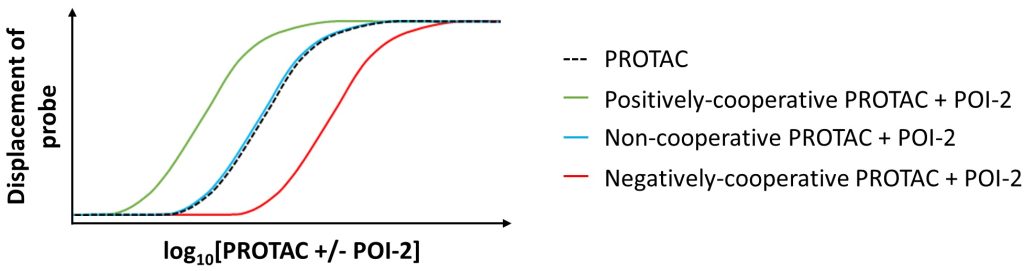
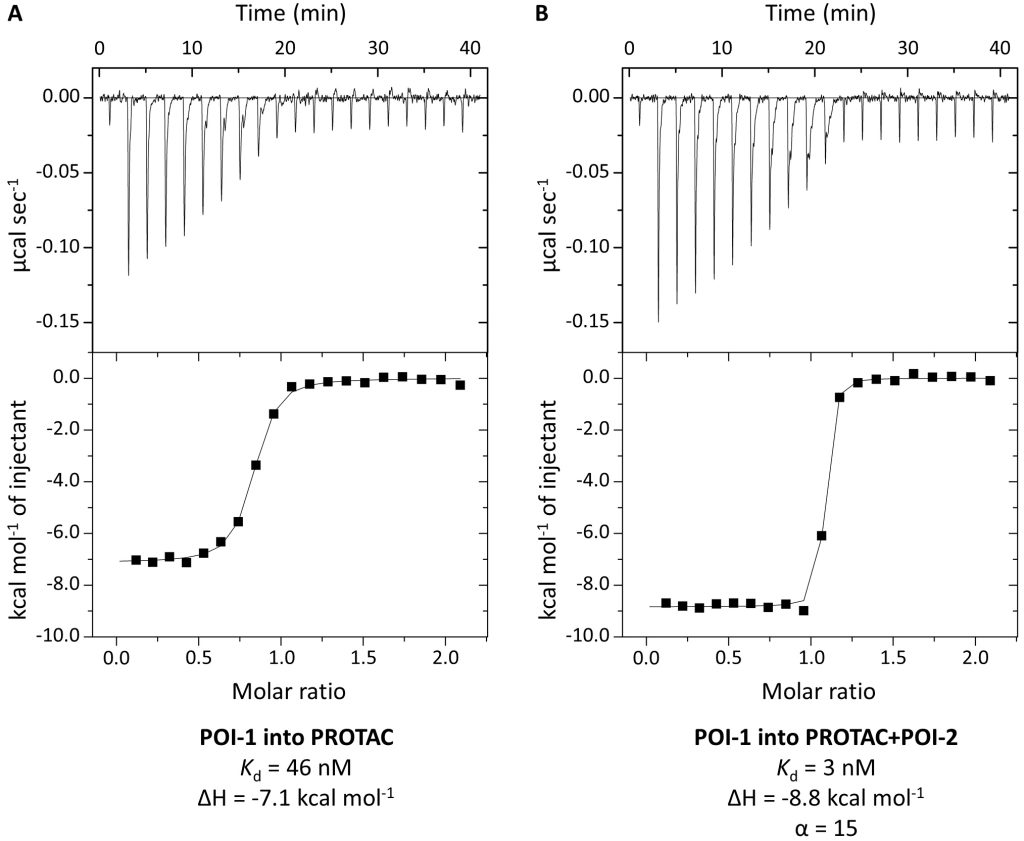
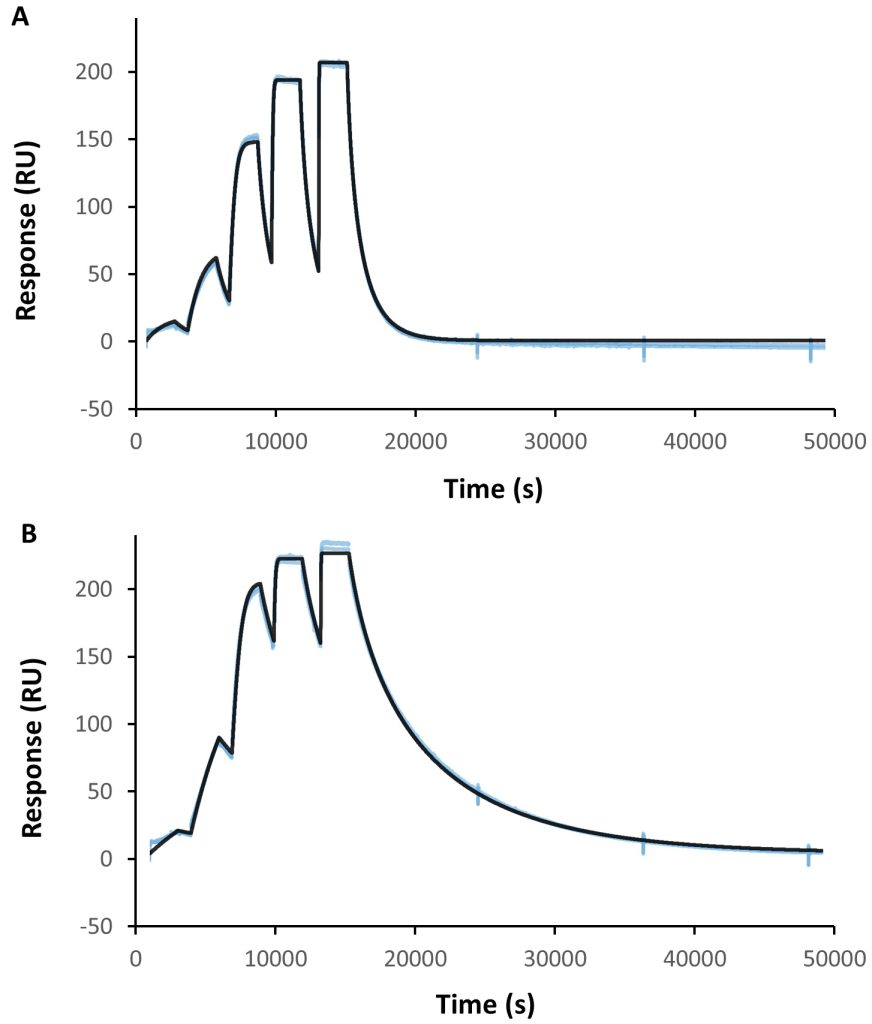
We illustrate how structural biology and biophysics are rapidly impacting the field and describe the main assays that are being developed and used to study PROTAC ternary complexes. The fundamental understanding that is emerging from these studies is beginning to illuminate important design principles that can now guide the field towards a more rational PROTAC design approach.