Our collaborative work on PROTAC structure-permeability relationships is now published in ACS Med Chem Lett.
Title: Understanding and Improving the Membrane Permeability of VH032-Based PROTACs
Authors: Victoria G. Klein, Chad E. Townsend, Andrea Testa, Michael Zengerle, Chiara Maniaci, Scott J. Hughes, Kwok-Ho Chan, Alessio Ciulli, and R. Scott Lokey
Abstract
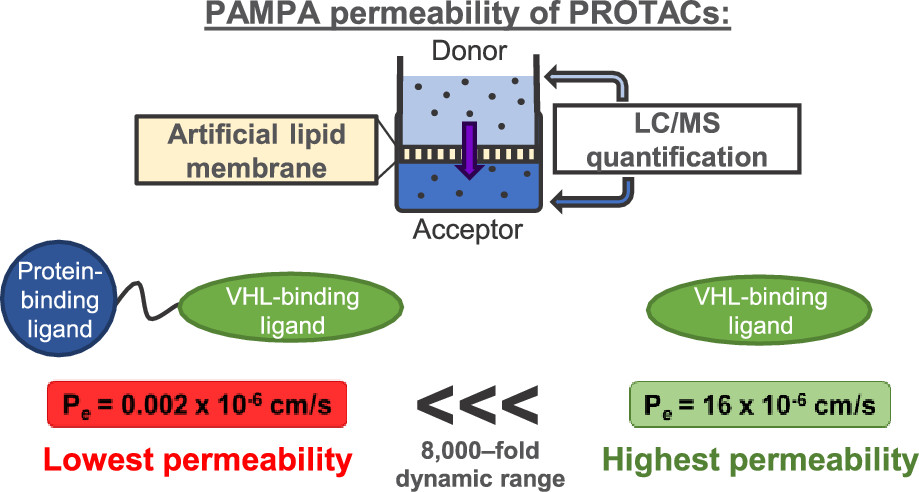
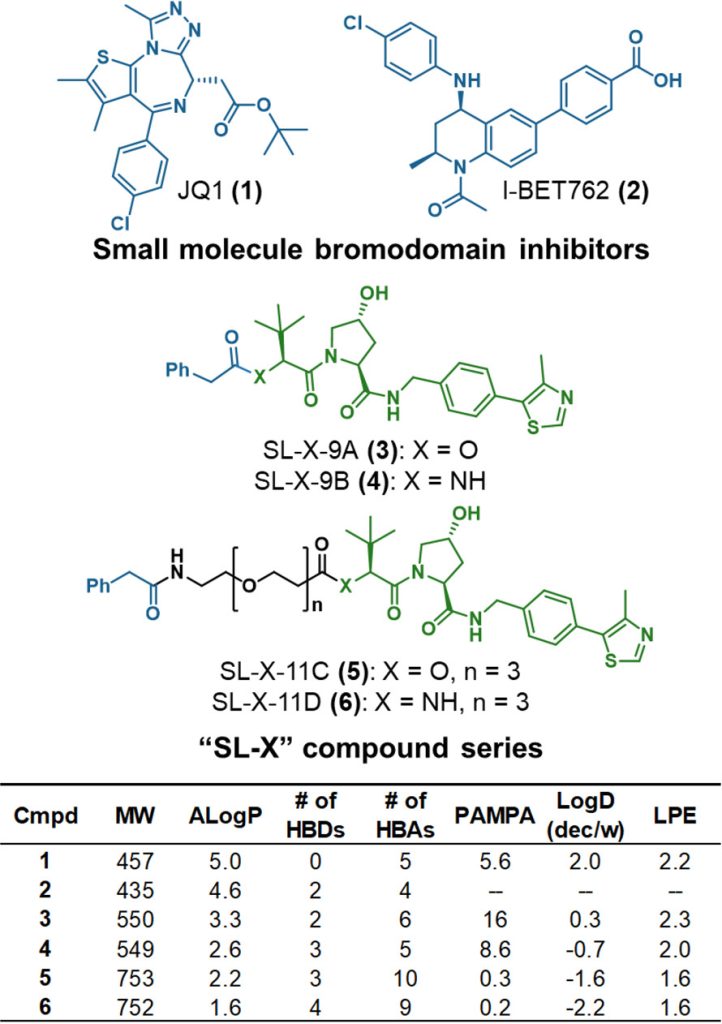
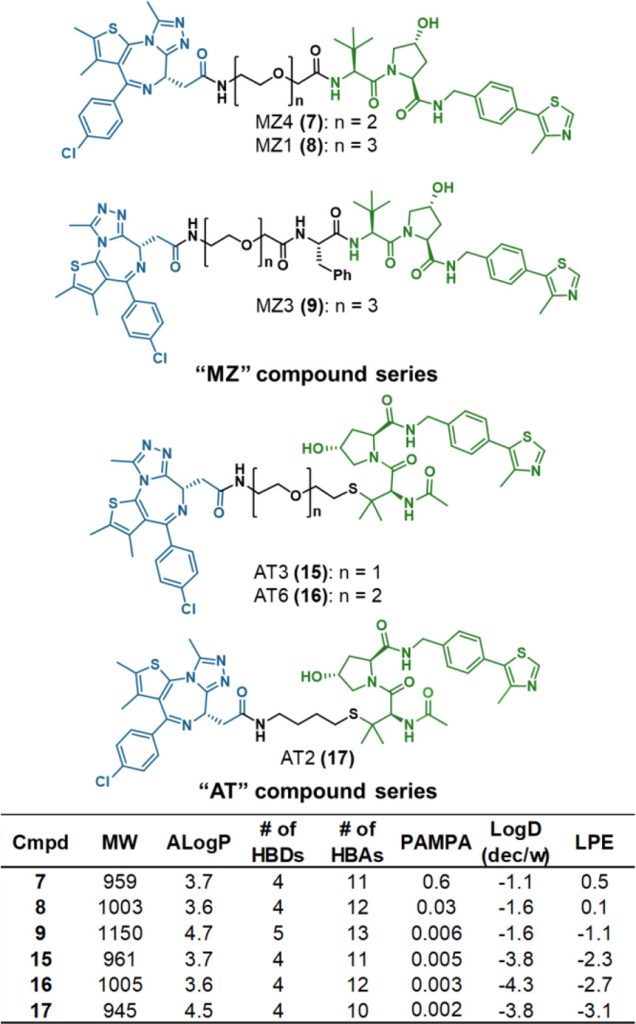
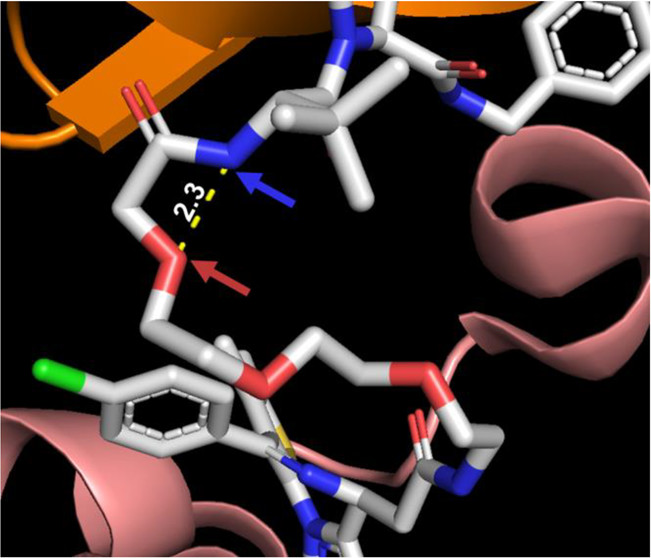
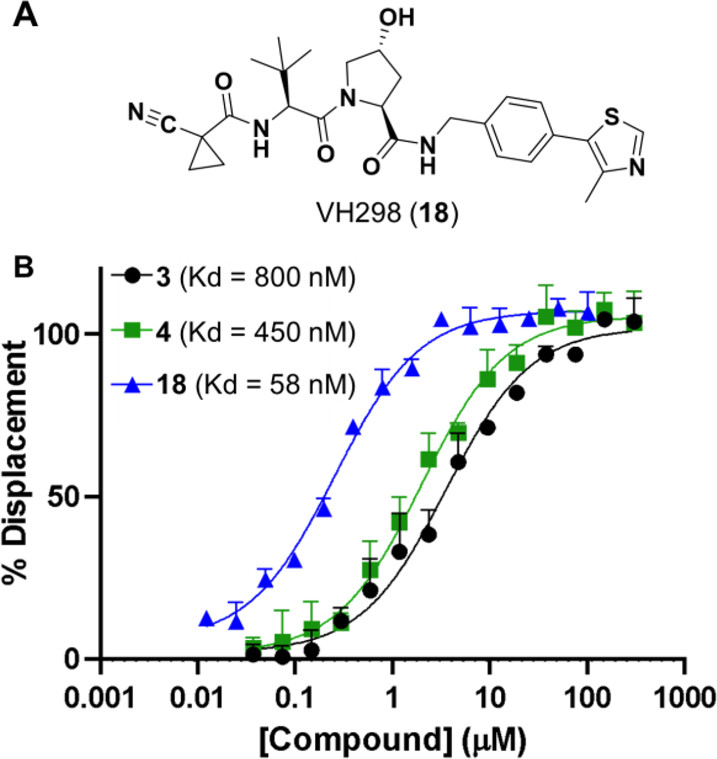
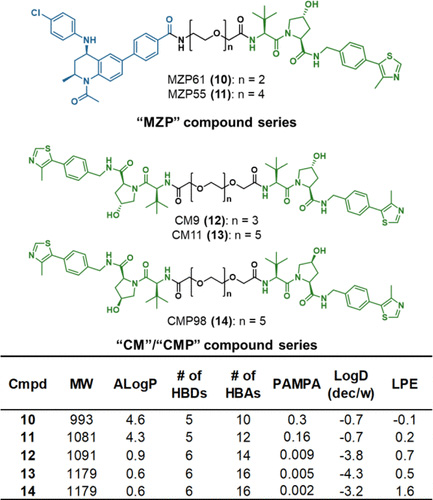
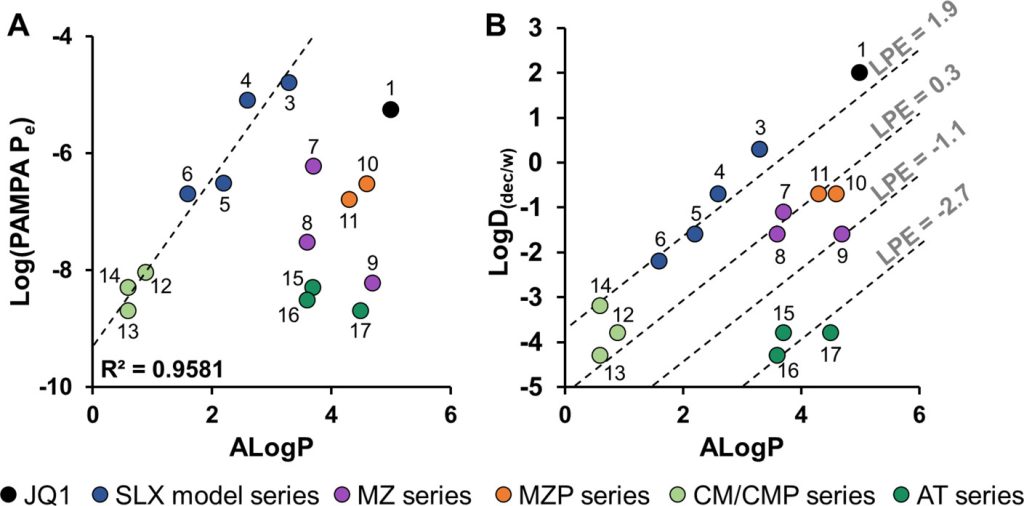
Proteolysis targeting chimeras (PROTACs) are catalytic heterobifunctional molecules that can selectively degrade a protein of interest by recruiting a ubiquitin E3 ligase to the target, leading to its ubiquitylation and degradation by the proteasome. Most degraders lie outside the chemical space associated with most membrane-permeable drugs. Although many PROTACs have been described with potent activity in cells, our understanding of the relationship between structure and permeability in these compounds remains limited. Here, we describe a label-free method for assessing the permeability of several VH032-based PROTACs and their components by combining a parallel artificial membrane permeability assay (PAMPA) and a lipophilic permeability efficiency (LPE) metric. Our results show that the combination of these two cell-free membrane permeability assays provides new insight into PROTAC structure–permeability relationships and offers a conceptual framework for predicting the physicochemical properties of PROTACs in order to better inform the design of more permeable and more effective degraders.
Congrats to Tori Klein in Scott Lokey’s lab for her work on our PROTAC molecules!