
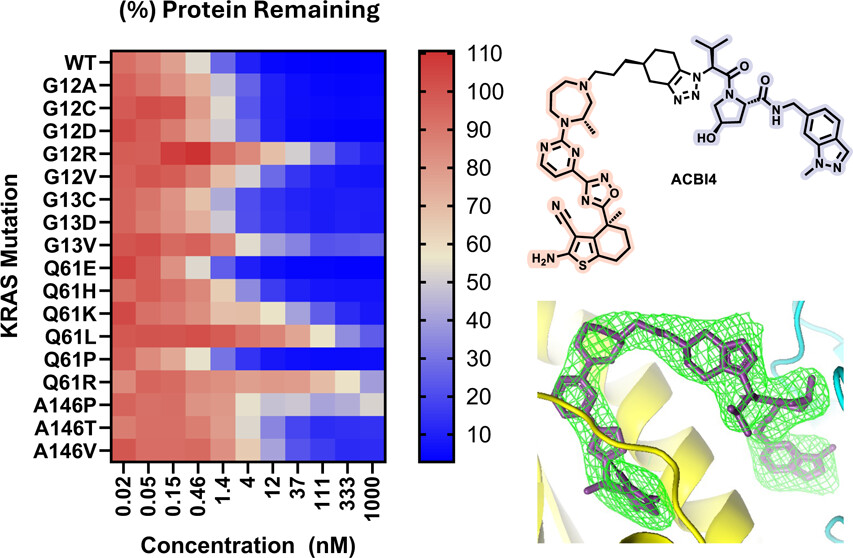
23. Identification of a Highly Cooperative PROTAC Degrader Targeting GTP-Loaded KRAS(On) Alleles
Vesna Vetma, Ilaria Puoti, Natalia K. Karolak, Sohini Chakraborti, Emelyne Diers, Enrico Girardi, Shakil Khan, Giorgia Kidd, Katrin G. Kropatsch, Ross Mclennan, Suzanne O’Connor, Matthias Samwer, Nicole Trainor, Claire Whitworth, Andre J. Wijaya, Jeff Y. F. Wong, David Zollman, William Farnaby, Johannes Popow, Alessio Ciulli*, Peter Ettmayer*, Kirsten McAulay*
J. Am. Chem. Soc. (2025)
doi: 10.1021/jacs.5c10354
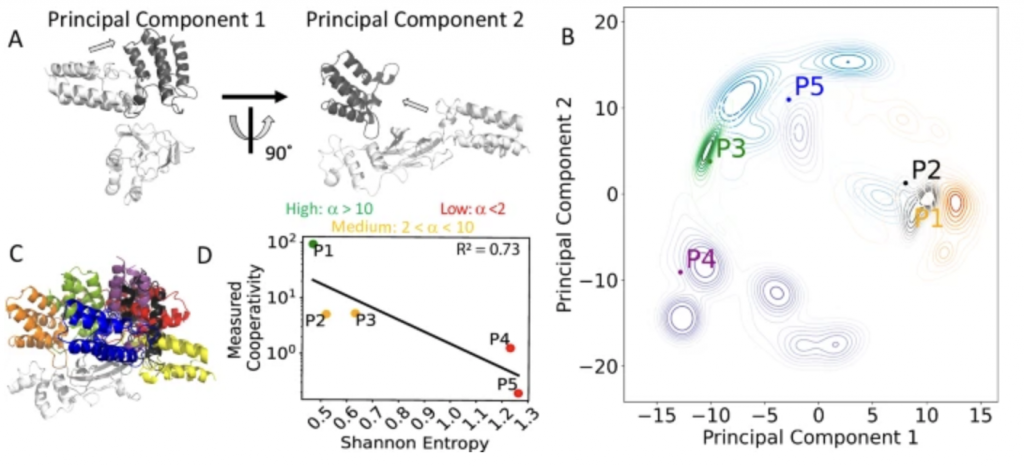
22. Frustration in the protein-protein interface plays a central role in the cooperativity of PROTAC ternary complexes
Ning Ma, Supriyo Bhattacharya, Sanychen Muk, Zuzana Jandova, Philipp S. Schmalhorst, Soumadwip Ghosh, Keith Le, Emelyne Diers, Nicole Trainor, William Farnaby, Michael J. Roy, Christiane Kofink, Peter Greb, Harald Weinstabl, Alessio Ciulli, Gerd Bader, Kyra Sankar, Andreas Bergner & Nagarajan Vaidehi
Nat Commun 16, 8595 (2025)
21. Stick it with VHL
William Farnaby
Nature Chemical Biology News and Views.
Publication date: 24th June 2025
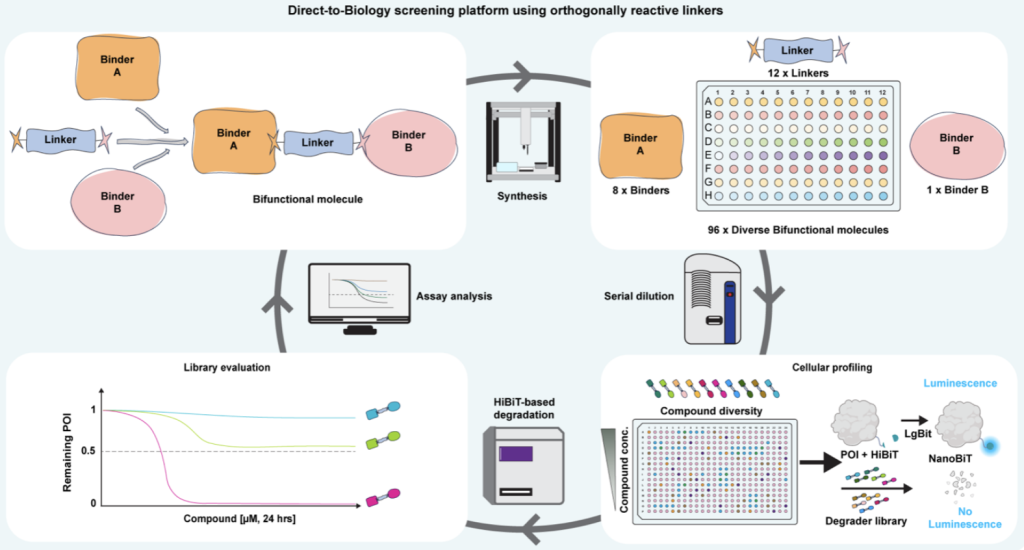
20. Discovery of a CNS active GSK3 degrader using orthogonally reactive linker screening
Andreas Holmqvist*, Nur Mehpare Kocaturk*, Christina Duncan, Jennifer Riley, Steve Baginski, Graham Marsh, Joel Cressor-Brown, Hannah Maple, Kristiina Juvonen, Gajanan Sathe, Nicola Morrice, Calum Sutherland, Kevin Read, William Farnaby (corresponding author)
Nat Commun 16, 8857 (2025)
doi: 10.1038/s41467-025-63928-8
Pre-print first posted at ChemRxiv on 10th March 2025; doi:: 10.26434/chemrxiv-2025-nq14w
*contributed equally as first author
19. Use of Aldehyde–Alkyne–Amine Couplings to Generate Medicinal Chemistry-Relevant Linkers
Andrew McGown*, Vesna Vetma, Damien Crepin, Yan Lin, Claire Adcock, Conner Craigon, Jordan Nafie, Daniel von Emloh, Léa Sutton, Kiera Bailey, Lewis Edmunds, Manvendra Sharma, Jonathan D. Wilden, Simon J. Coles, Graham J. Tizzard, William Farnaby, Alessio Ciulli, George E. Kostakis, John Spencer*
ACS Med. Chem. Lett. Publication date : January 25th, 2025
doi: 10.1021/acsmedchemlett.4c00531
*co-corresponding authors
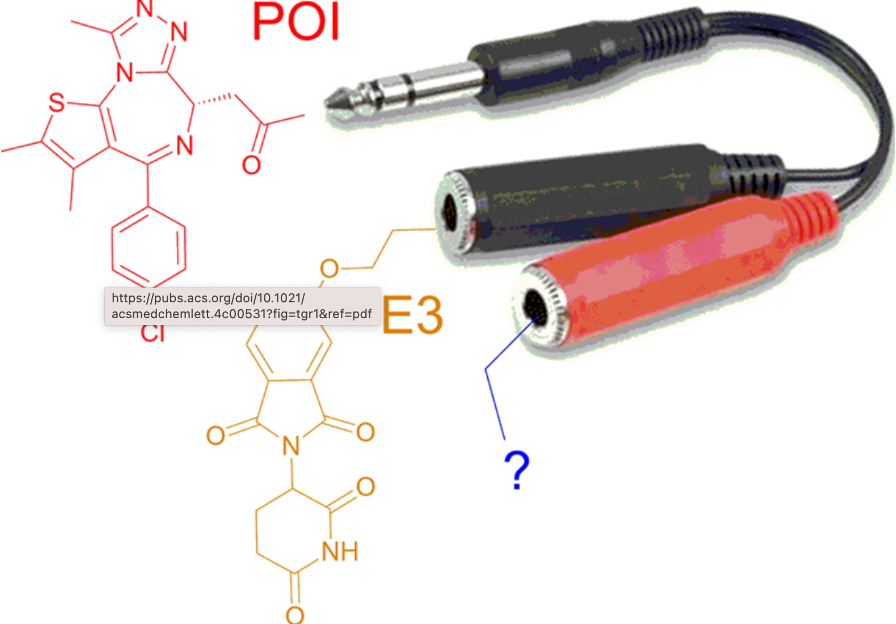
18. Targeting cancer with small molecule pan-KRAS degraders
Johannes Popow*, William Farnaby*, Andreas Gollner*, Christiane Kofink*, Gerhard Fischer, Melanie Wurm, David Zollman, Andre Wijaya, Nikolai Mischerikow, Carina Hasenoehrl, Polina Prokofeva, Heribert Arnhof, Silvia Arce-Solano, Sammy Bell, Georg Boeck, Emelyne Diers, Aileen B Frost, Jake Goodwin-Tindall, Jale Karolyi-Oezguer, Shakil Khan, Theresa Klawatsch, Manfred Koegl, Roland Kousek, Barbara Kratochvil, Katrin Kropatsch, Arnel A Lauber, Ross McLennan, Sabine Olt, Daniel Peter, Oliver Petermann, Vanessa Roessler, Peggy Stolt-Bergner, Patrick Strack, Eva Strauss, Nicole Trainor, Vesna Vetma, Claire Whitworth, Siying Zhong, Jens Quant, Harald Weinstabl, Bernhard Küster, Peter Ettmayer (lead/corresponding author), Alessio Ciulli (lead/corresponding author)
*contributed equally as first author
Science. Publication Date: September 19, 2024, doi: 10.1126/science.adm8684
Pre-print first posted at bioRxiv on 26th October 2023; doi: 10.1101/2023.10.24.563163
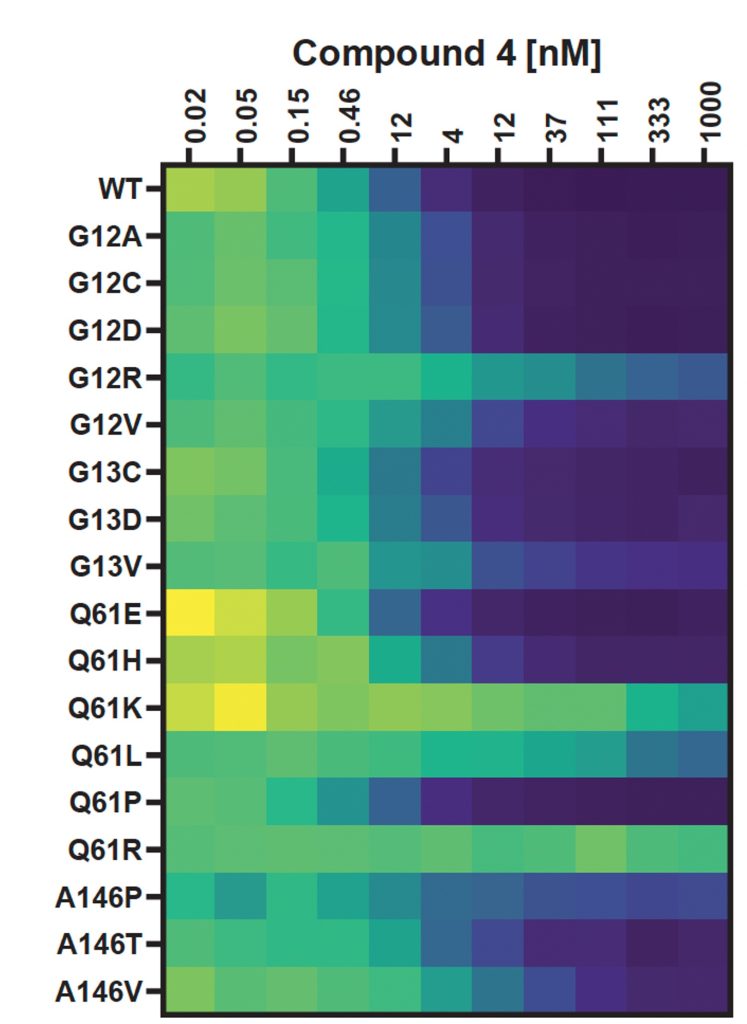
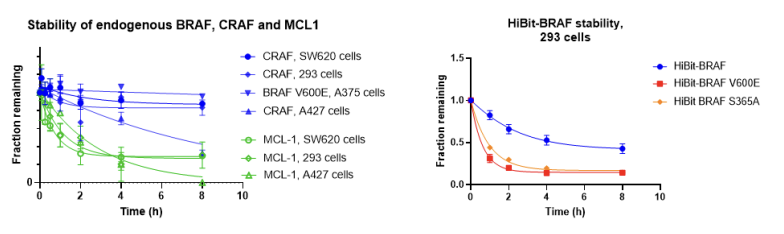
17. Confounding factors in targeted degradation of short-lived proteins
Vesna Vetma, Laura Casares-Perez, Ján Eliaš, Andrea Stingu, Anju Kombara, Teresa Gmaschitz, Nina Braun, Tuncay Ciftci, Georg Dahmann, Emelyne Diers, Thomas Gerstberger, Peter Greb, Giorgia Kidd, Christiane Kofink, Ilaria Puoti, Valentina Spiteri, Nicole Trainor, Yvonne Westermaier, Claire Whitworth, Alessio Ciulli, William Farnaby, Kirsten McAulauy, Aileen B. Frost, Nicola Chessum, Manfred Koegl
ACS Chem. Biol. Publication Date: July 3, 2024, doi: 10.1021/acschembio.4c00152
Pre-print first posted at BioRxiv on 22 February 2024; doi: 10.1101/2024.02.19.581012
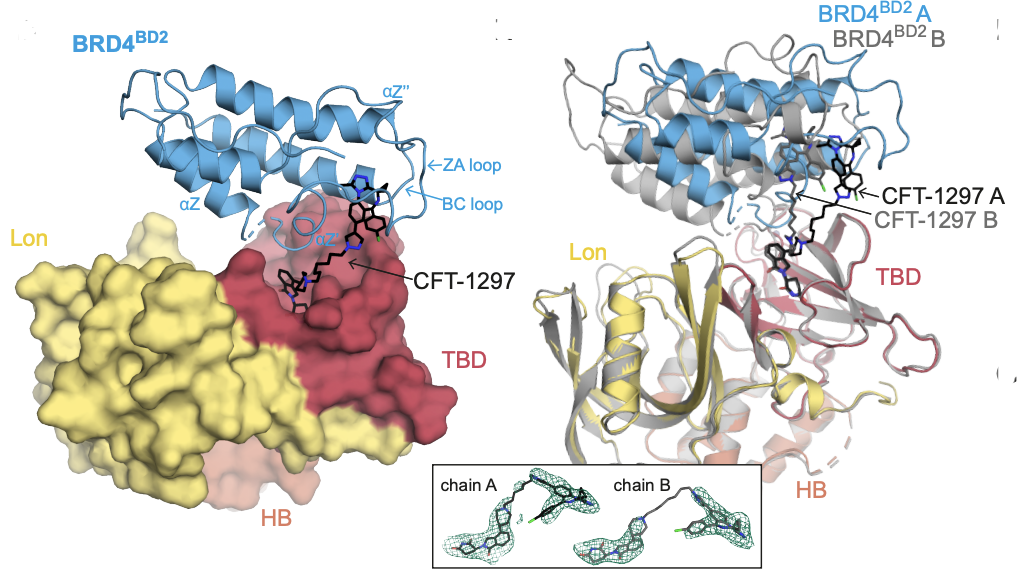
16. Design of a Cereblon construct for crystallographic and biophysical studies of protein degraders
Alena Kroupova, Valentina A. Spiteri, Hirotake Furihata, Darren Darren, Sarath Ramachandran, Zoe J. Rutter, Sohini Chakraborti, Kevin Haubrich, Julie Pethe, Denzel Gonzales, Andre Wijaya, Maria Rodriguez-Rios, Dylan M. Lynch, William Farnaby, Mark A. Nakasone, *David Zollman, *Alessio Ciulli
*co-corresponding authors
Nat Commun. Publication date 15th October 2024;
doi: 10.1038/s41467-024-52871-9
Pre-print first posted at BioRxiv on 20 January 2024
15. Breaking free from the crystal lattice: Structural biology in solution to study protein degraders.
Kevin Haubrich, Valentina Spiteri, William Farnaby, Frank Sobott, Alessio Ciulli
Curr Opin Struct Biol 2023, 79, 10234.

14. Crystallization of VHL-based PROTAC-induced ternary complexes
Andre J. Wijaya, William Farnaby, Alessio Ciulli
Methods Enzymol 2023, 681, 242-261.
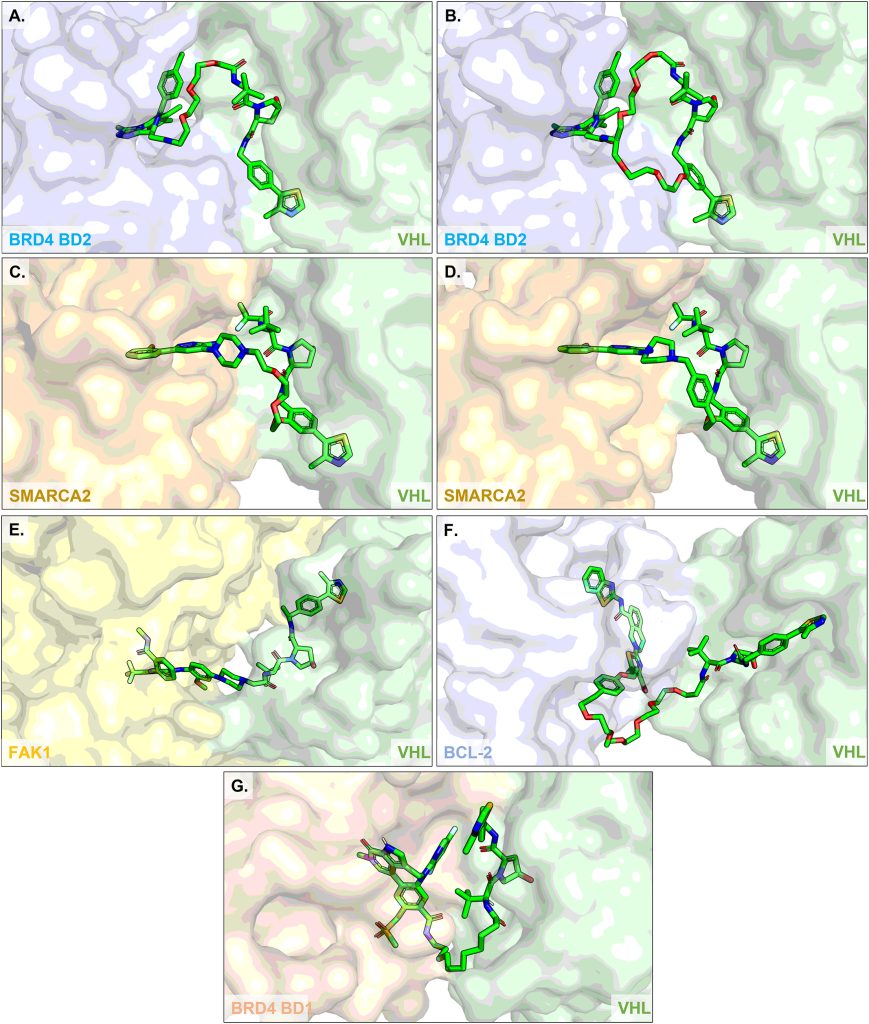
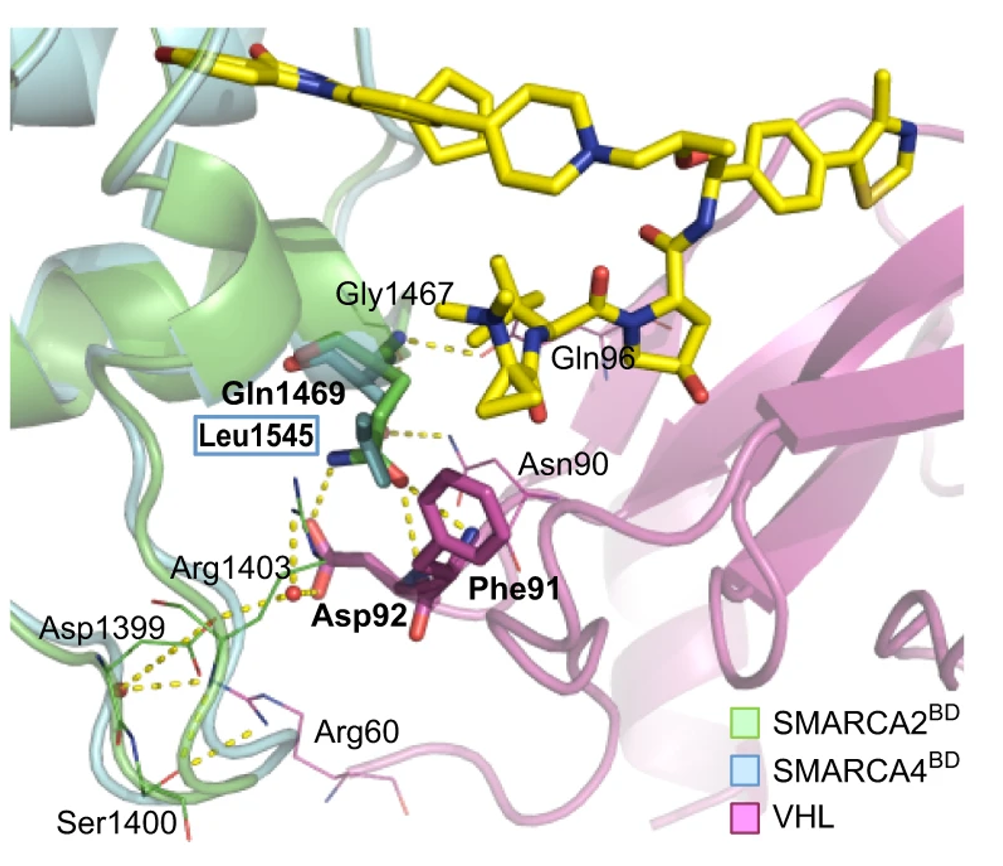
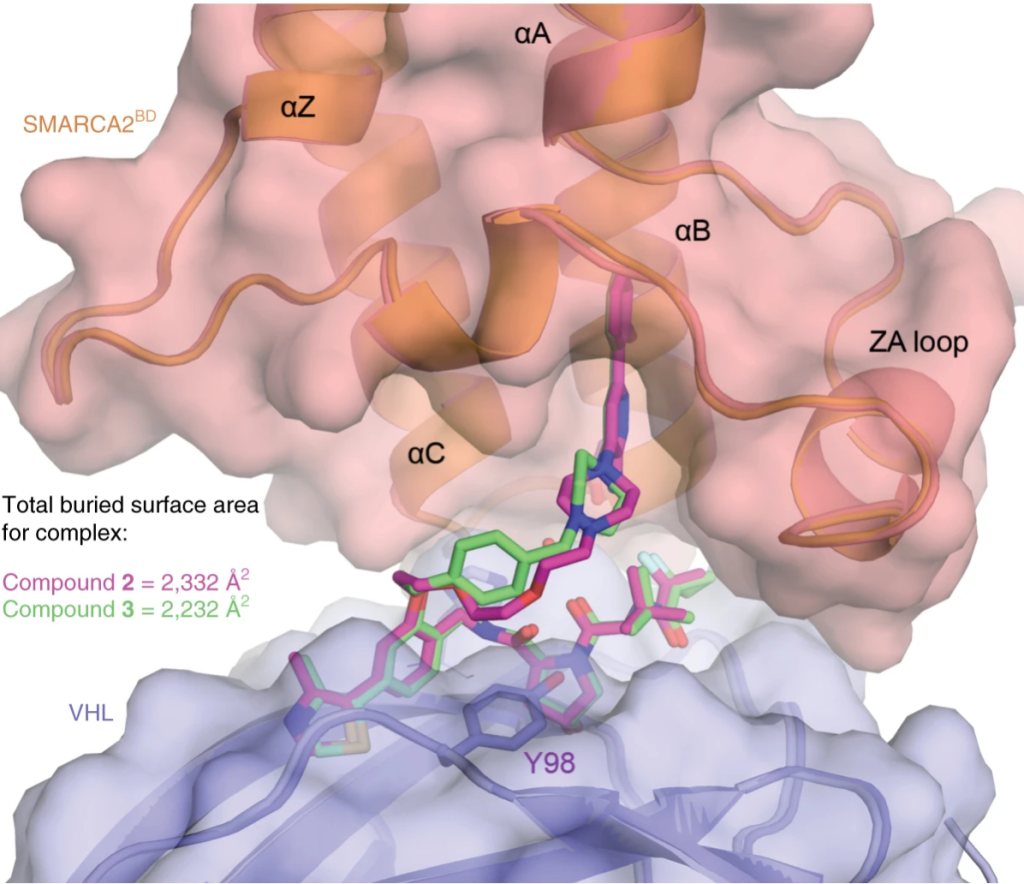
13. A selective and orally bioavailable VHL-recruiting PROTAC achieves SMARCA2 degradation in vivo
Christiane Kofink, Nicole Trainor, Barbara Mair, Simon Wöhrle, Melanie Wurm, Nikolai Mischerikow, Michael J. Roy, Gerd Bader, Peter Greb, Géraldine Garavel, Emelyne Diers, Ross McLennan, Claire Whitworth, Vesna Vetma, Klaus Rumpel, Maximilian Scharnweber, Julian E. Fuchs, Thomas Gerstberger, Yunhai Cui, Gabriela Gremel, Paolo Chetta, Stefan Hopf, Nicole Budano, Joerg Rinnenthal, Gerhard Gmaschitz, Moriz Mayer, Manfred Koegl, Alessio Ciulli, Harald Weinstabl & William Farnaby
Nat. Commun. 2022, 13 (1), 5969.
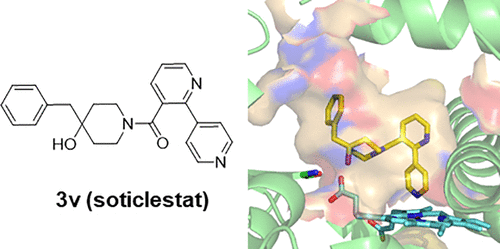
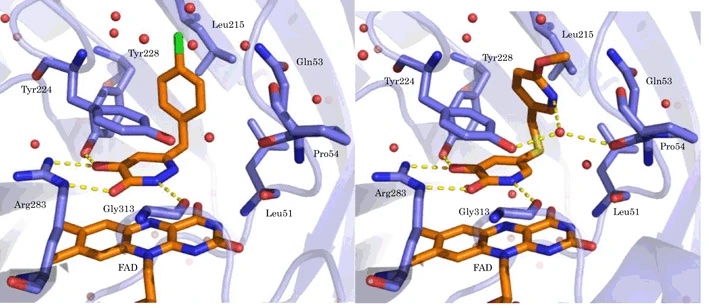
12. Discovery of Soticlestat, a Potent and Selective Inhibitor for Cholesterol 24-Hydroxylase (CH24H)
Tatsuki Koike, Masato Yoshikawa, Haruhi Kamisaki Ando, William Farnaby, Toshiya Nishi, Etsorou Watanabe, Jason Yano, Maki Miyamoto, Shigeru Kondo, Tsuyoshi Ishi, Takanobu Kuroita
J Med Chem 2021 64(16):12228-12244.
11. Transforming targeted cancer therapy with PROTACs: A forward-looking perspective
William Farnaby, Manfred Koegl, Darryl B. McConnell, Alessio Ciulli
Curr. Opin. Pharmacol. 2021, 57, 175-183

10. Soticlestat, a novel cholesterol 24-hydroxylase inhibitor shows a therapeutic potential for neural hyperexcitation in mice
Toshiya Nishi, Shinichi Kondo, Maki Miyamoto, Sayuri Watanabe, Shigeo Hasegawa, Shigeru Kondo, Jason Yano, Etsurou Watanabe, Tsuyoshi Ishi, Masato Yoshikawa, Haruhi Kamisaki Ando, William Farnaby, Shinji Fujimoto, Eiji Sunahara, Momoko Ohori, Matthew J. During, Takanobu Kuroita & Tatsuki Koike
Scientific Reports 2020, 10, 17081

9. Protein degradation for drug discovery. [Editorial]
Alessio Ciulli & William Farnaby
Drug Discov. Today Technol. 2019 Apr 31: 1-3
8. BAF complex vulnerabilities in cancer demonstrated via structure-based PROTAC design
William Farnaby*, Manfred Koegl*, Michael J. Roy, Claire Whitworth, Emelyne Diers, Nicole Trainor, David Zollman, Steffen Steurer, Jale Karolyi-Oezguer, Carina Riedmueller, Teresa Gmaschitz, Johannes Wachter, Christian Dank, Michael Galant, Bernadette Sharps, Klaus Rumpel, Elisabeth Traxler, Thomas Gerstberger, Renate Schnitzer, Oliver Petermann, Peter Greb, Harald Weinstabl, Gerd Bader, Andreas Zoephel, Alexander Weiss-Puxbaum, Katharina Ehrenhöfer-Wölfer, Simon Wöhrle, Guido Boehmelt, Joerg Rinnenthal, Heribert Arnhof, Nicola Wiechens, Meng-Ying Wu, Tom Owen-Hughes, Peter Ettmayer, Mark Pearson, Darryl B. McConnell & Alessio Ciulli
*contributed equally
Nat Chem Biol 2019, 15 (7), 672–680.
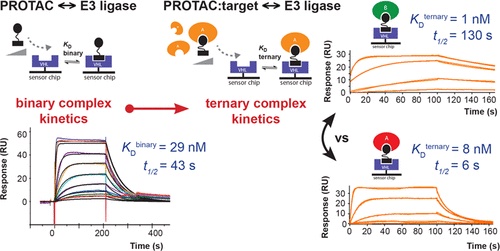
7. SPR-measured dissociation kinetics of PROTAC ternary complexes influence target degradation rate
Michael J. Roy, Sandra Winkler, Scott J. Hughes, Claire Whitworth, Michael Galant, William Farnaby, Klaus Rumpel, Alessio Ciulli
6. Identification of compounds acting as negative allosteric modulators of the LPA1 receptor
Jonathan Ellery, Louise Dickson, Toni Cheung, Loredana Ciuclan, Peter Bunyard, Stephen Mack, William J Buffham, William Farnaby, Philip Mitchell, Daniel Brown, Richard Isaacs, Matt Barnes
Eur J Pharmacol 2018 833, 8-15.
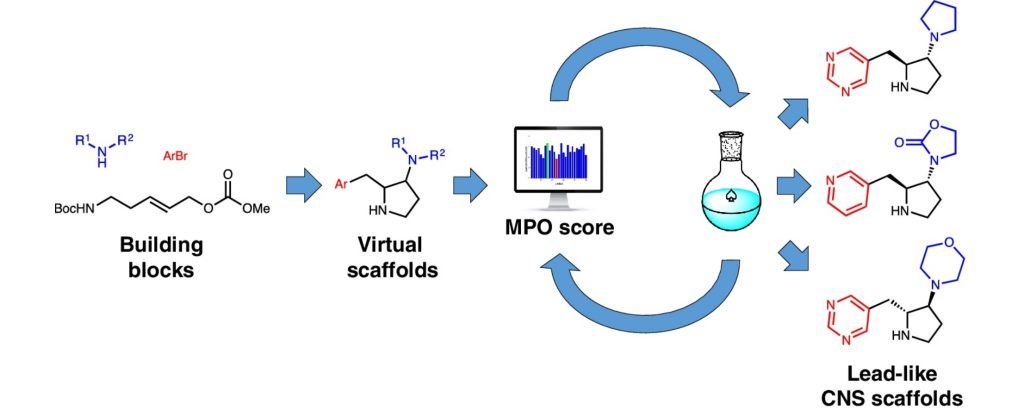
5. Modular synthesis of thirty lead-like scaffolds suitable for CNS drug discovery
Joan Mayol-Llinas, William Farnaby, Adam Nelson
Chem. Commun. 2017, 53, 12345-12348
doi: 10.1039/C7CC06078E

4. Assessment of the Target Engagement and D-Serine Biomarker Profiles of the D-Amino Acid Oxidase Inhibitors Sodium Benzoate and PGM030756
Eimear Howley, Michael Bestwick, Rosa Fradley, Helen Harrison, Mathew Leveridge, Kengo Okada, Charlotte Fieldhouse, Will Farnaby, Hannah Canning, Andy P Sykes, Kevin Merchant, Katherine Hazel, Catrina Kerr, Natasha Kinsella, Louise Walsh, David G Livermore, Isaac Hoffman, Jonathan Ellery, Phillip Mitchell, Toshal Patel, Mark Carlton, Matt Barnes, David J Miller
Neurochem Res 2017, 42, 3279-3288.
3. Assessing molecular scaffolds for CNS drug discovery
Joan Mayol-Llinas, Adam Nelson, William Farnaby, Andrew Ayscough
Drug Discov Today 2017, 22, 965-969.
2. Synthesis of (−)-(S,S)-clemastine by Invertive N → C Aryl Migration in a Lithiated Carbamate
Anne M. Fournier, Robert A. Brown, William Farnaby, Hideki Miyatake-Ondozabal and Jonathan Clayden
Org. Lett. 2010, 12, 10, 2222–2225
doi: 10.1021/ol100627c

1. N to C Aryl Migration in Lithiated Carbamates: α-Arylation of Benzylic Alcohols
Jonathan Clayden, William Farnaby, Damian M. Grainger, Ulrich Hennecke, Michele Mancinelli ,Daniel J. Tetlow, Ian H. Hillier and Mark A. Vincent
J Am Chem Soc 2009, 131, 3410-3411.
doi: 10.1021/ja808959e

Patents
(Several have been granted in varying territories; for simplicity initial applications are shown only)
1. KRAS Degrading compounds comprising annulated 2-amino-3-cyano thiophenes WO2023099620 (2023)
2. Proteolysis targeting chimera (Protacs) as degraders of SMARCA2 and/or SMARCA4. WO2020078933 (2020)
3. 3-Substituted 2-aminoazaindole derivatives as GPR43 agonists and positive allosteric modulators and their preparation. WO2015198045 (2015).
4. Preparation of 1,3-substituted 2-aminoindole derivatives and analogues useful in the treatment or prevention of diabetes mellitus, obesity and inflammatory bowel disease. WO2015198046 (2015).
5. Preparation of amide derivatives as lysophosphatidic acid receptor antagonists. WO2015025164 (2015).
6. Preparation of pyridine and pyrimidine derivatives as cholesterol 24 hydroxylase inhibitors. WO2014163161 (2014).
7. Pyridazinones as DAAO enzyme inhibitors. WO2014096757 (2014)
8. Pyridazinone compounds and their use as DAAO inhibitors. WO2013027000 (2013).
9. Preparation of substituted hydroxypiperidines as cholesterol 24-hydroxylase inhibitors. US20130090341 (2013).
10. 5- or 6- substituted 3-hydroxy-2-(1H)-pyridinones as d-amino acid oxidase (DAAO) inhibitors in therapy of diseases such as schizophrenia, cognitive disorder and pain. WO2013004996 (2013).
11. Pyrimidinone compounds and their use. WO2013004995 (2013).