
 BACKGROUND:
BACKGROUND:
The Bronchiectasis Research Involving Databases, Genomics and Endotyping (BRIDGE) study represents an observational, multicentre, mechanistic sub-study conducted through the EMBARC consortium (NCT03791086) (Aliberti et al., 2018).
STUDY AIMS & OBJECTIVES:
The study’s primary aim is to phenotype and endotype bronchiectasis patients during stable disease and periods of exacerbation to delineate the underlying mechanisms driving disease, with the hope of developing strategies for personalised medicine and guiding treatment response.
The BRIDGE study also has the following secondary aims:
- To identify molecular endotypes of bronchiectasis during periods of disease stability.
- To identify the causes of, and better understand the inflammatory component of, bronchiectasis exacerbations.
- To identify and validate biomarkers of disease with potential use in personalised medicine.
- To generate proof-of-concept data by in vivo and in vitro studies to identify subsets of patients likely to benefit in upcoming randomised controlled trials.
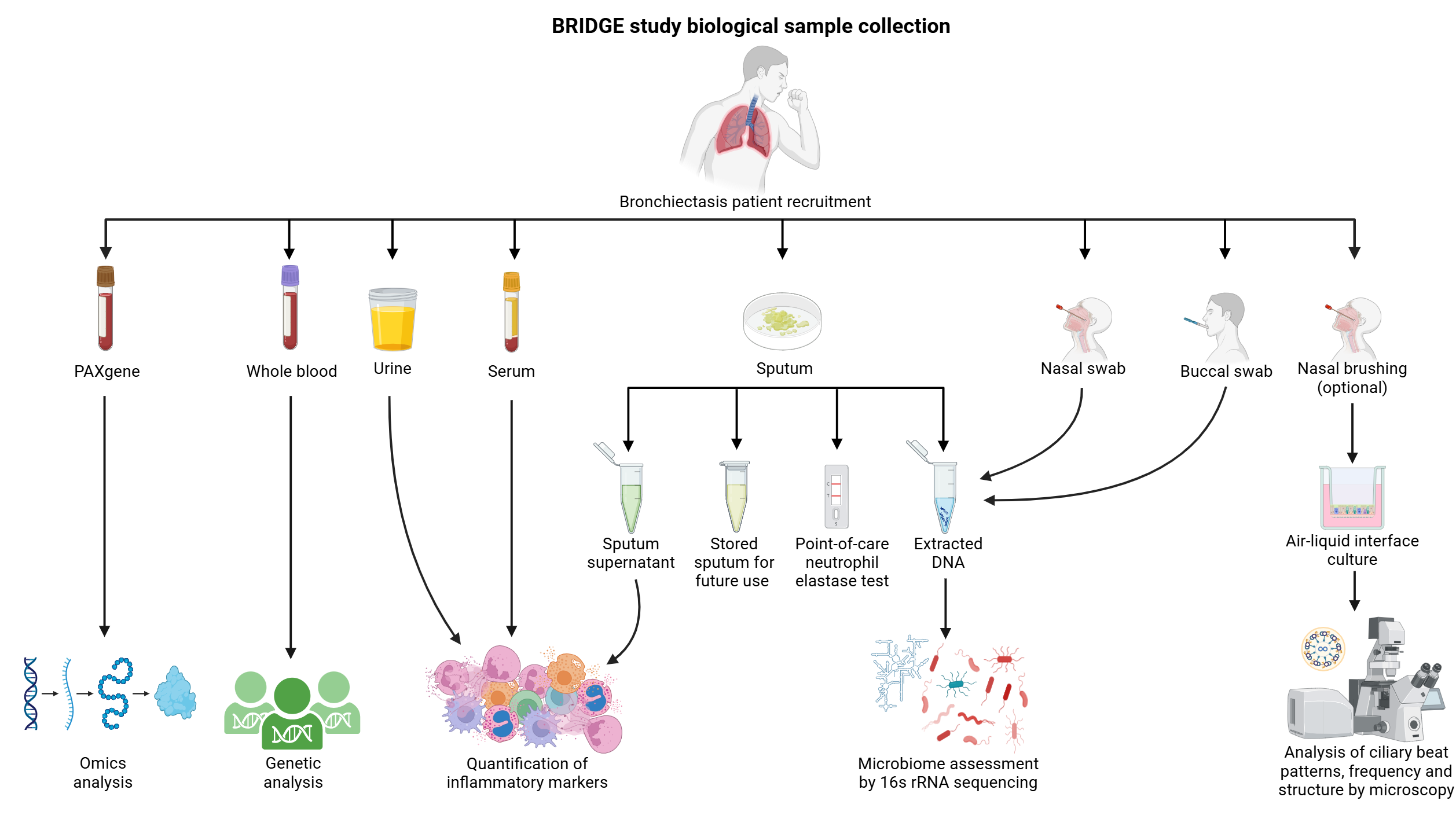
SAMPLE COLLECTION:
BRIDGE collects both clinical data and biological samples at recruitment with annual (± 3 months) follow-up data collected for up to 3 years following initial recruitment – 4 study visits in total.
A summary of the biological samples collected are shown below.
STUDY RECRUITMENT:
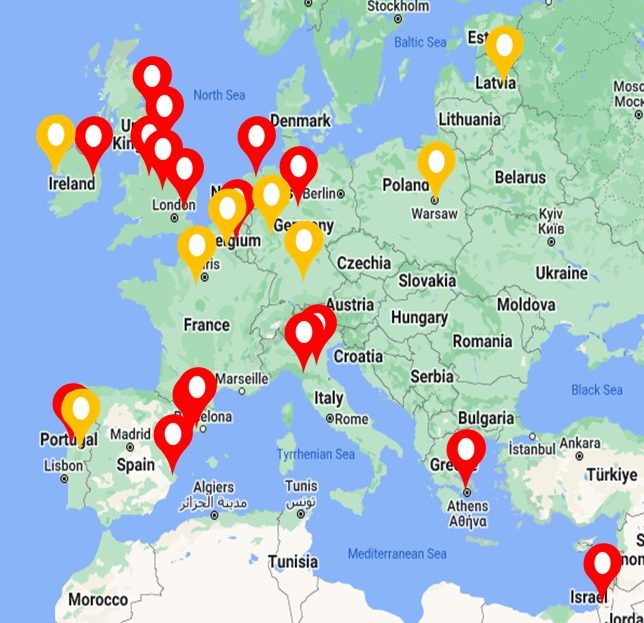
Patient enrolment into the BRIDGE study began in January 2019 with recruitment currently ongoing (NCT03791086).
As of February 2025, we have recruited 1,476 patients into BRIDGE.
We currently have 26 research sites taking part in the EMBARC-BRIDGE study (red), with additional sites in set up (yellow).
These include:
UK (Dundee, Fife, Newcastle, Manchester x 2, London, Cambridge, Bristol x 2)
Ireland (Dublin)
Italy (Milan x 2)
Spain (Barcelona x 2, Valencia)
Portugal (Porto, Gondomar)
Germany (Hannover, Muenster)
The Netherlands (Amsterdam)
Belgium (Leuven)
Greece (Athens)
Israel (Haifa)
South Korea (Seoul)
North Macedonia
Poland (Warsaw)
 If you are a research site interested in contributing data/samples to the EMBARC-BRIDGE study, please register your interest here.
If you are a research site interested in contributing data/samples to the EMBARC-BRIDGE study, please register your interest here.
BRIDGE Protocol Receipt V4 24.01.24
BRIDGE Letter of invitation V2 26.01.24
BRIDGE Participant Information Sheet V3 24.01.24
BRIDGE Informed Consent Form V2 21.03.24
BRIDGE Cilia sub-study Participation Information Sheet V2 24.01.24
BRIDGE Cilia sub-study Informed Consent Form V2 24.01.24
BRIDGE GP and Clinic Letter V2 26.01.24
BRIDGE BIM Baseline questionnaire, V2.0, 24-04-2019
BRIDGE BIM Follow-up questionnaire, V2.0, 24-04-2019
Questionnaire QOL-B, Scoring Instructions V1 12-09-2018
