ESCAPE - A Randomised Trial of Pseudomonas Eradication
 BACKGROUND:
BACKGROUND:
Approximately 1/3 of bronchiectasis patients become chronically infected with the opportunistic bacteria, Pseudomonas aeruginosa (P. aeruginosa), which contributes to increased disease severity, poorer clinical outcomes and worsened quality of life.
Once infection with P. aeruginosa infection is established, it is almost impossible to clear due to the formation of dense biofilms and adaptations in the lung that favour immune evasion. In the early stages of infection patients may feel well but it is believed that there is a “window of opportunity” following initial acquisition of infection to eradicate the infection with antibiotics.
Consequently treatment guidelines make conditional recommendations for Pseudomonas eradication by providing a combination of systemic (oral or intravenous) and inhaled antibiotics for 3 months. However, there are no large randomized controlled trials proving that this approach is effective, safe or cost-effective.
TRIAL OVERVIEW:
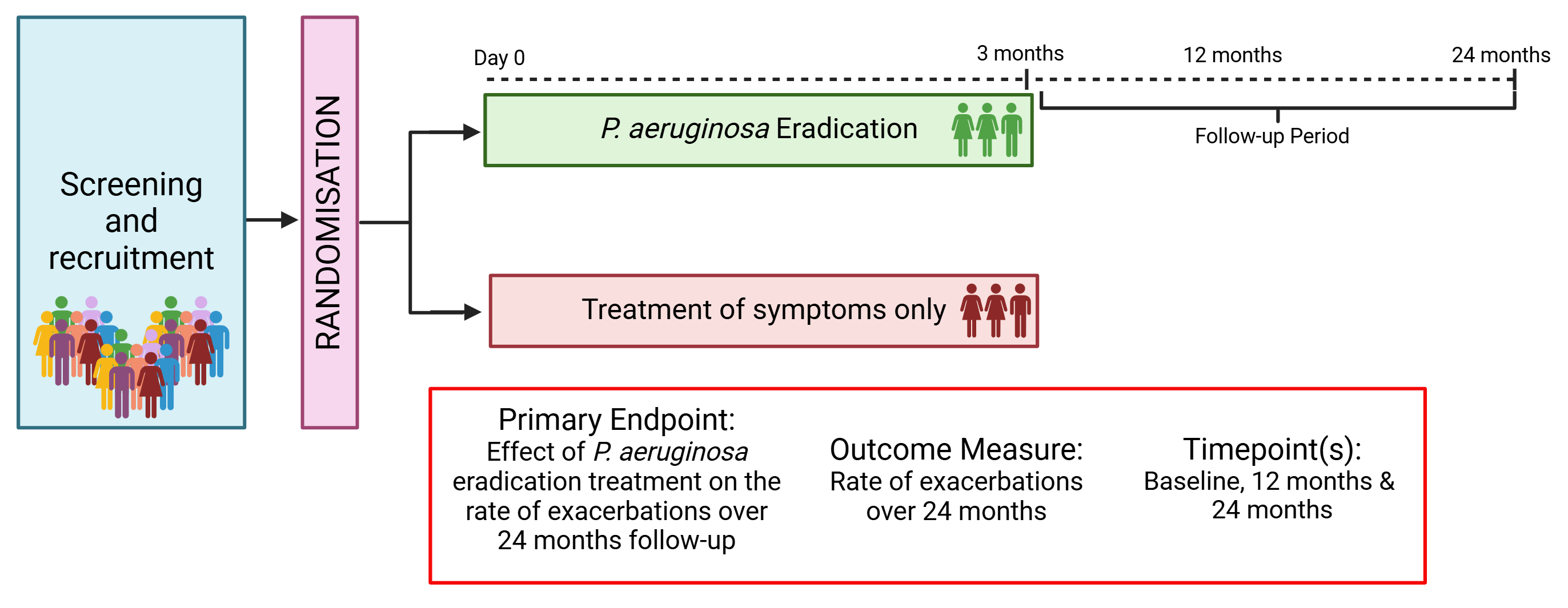
ESCAPE is a pragmatic, randomised, multi-centre, open-label clinical trial testing the effect of P. aeruginosa eradication treatment (compared to treatment for symptoms only) in those with bronchiectasis with a new isolation of P. aeruginosa.
The ESCAPE trial aims to:
To estimate the effects of 3 months eradication treatment vs symptomatic treatment alone in bronchiectasis patients with new P. aeruginosa infection
To determine the safety and cost-effectiveness of P. aeruginosa eradication treatment in bronchiectasis patients.
To work in partnership with patients to deliver a patient centred clinical trial and to achieve optimal study design, recruitment and dissemination.
The trial is not blinded, meaning participants will know which treatment they are receiving.
 This trial is not yet recruiting.
This trial is not yet recruiting.
The ESCAPE Trial is managed by the Tayside Clinical Trials Unit (TCTU).
Further information on the trial, including essential trial documentation and training resources, will be provided when the trial is recruiting.
