
BACKGROUND:
 Neutrophilic inflammation is a dominant part of the inflammatory process in bronchiectasis, with increased levels frequently associated with disease severity, worsened clinical outcomes and reduced quality of life. Attenuating neutrophilic inflammation by inhibition of dipeptidyl peptidase-1 (DPP-1) has been shown to reduce exacerbation frequency and prolong the time-to-first exacerbation in the Phase II WILLOW trial (Chalmers et al., 2020), a finding which has recently been validated in the Phase III ASPEN trial (topline data announced in 2024). The WILLOW trial also confirming sputum Neutrophil Elastase as a valid biomarker for use in other clinical trials of anti-inflammatory therapies in bronchiectasis.
Neutrophilic inflammation is a dominant part of the inflammatory process in bronchiectasis, with increased levels frequently associated with disease severity, worsened clinical outcomes and reduced quality of life. Attenuating neutrophilic inflammation by inhibition of dipeptidyl peptidase-1 (DPP-1) has been shown to reduce exacerbation frequency and prolong the time-to-first exacerbation in the Phase II WILLOW trial (Chalmers et al., 2020), a finding which has recently been validated in the Phase III ASPEN trial (topline data announced in 2024). The WILLOW trial also confirming sputum Neutrophil Elastase as a valid biomarker for use in other clinical trials of anti-inflammatory therapies in bronchiectasis.
The success of targeting inflammation in bronchiectasis has generated increased interest in identifying additional anti-inflammatory therapies for use in bronchiectasis. Interestingly, numerous existing drugs show secondary anti-inflammatory properties beyond their original indication which have relevance in bronchiectasis, and their widespread use in other conditions has generated a established evidence base regarding their safety in different patient populations. As such, there is a strong desire to explore these drugs as novel anti-inflammatory therapies in bronchiectasis.
Adaptive platform trials – including multi-arm, multi-stage trials – offer the potential to efficiently evaluate the therapeutic effect of multiple potential treatment options in a more cost- and time-efficient manner. Platform trials allow drugs to be screened out if they appear ineffective and newly-identified drugs that show therapeutic potential to be added in, with drugs showing a promising efficacy signal and acceptable safety profile being taken forward into a larger randomised Phase III trial.
TRIAL OVERVIEW:
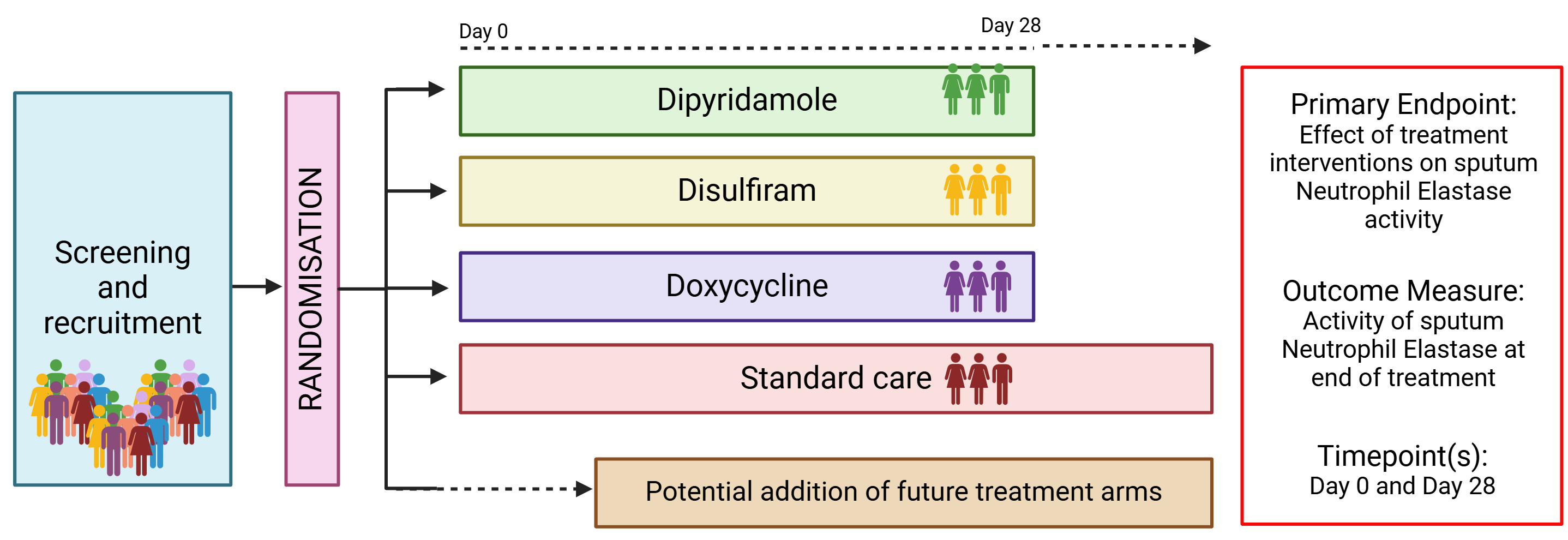
AIR-NET is a randomised, open-label, multi-arm, multi-centre Phase II adaptive platform trial designed to test the anti-inflammatory effects of multiple repurposed interventions (versus standard care) in patients with bronchiectasis.
The drugs currently being tested for their neutrophil-targeting, anti-inflammatory properties in bronchiectasis include:
- Doxycycline – approved for use as an antibiotic.
- Disulfiram – approved for use as an alcohol dehydrogenase inhibitor to treat alcohol dependency.
- Dipyridamole – approved for use to prevent blood clot formation.
 The AIR-NET trial is managed by Tayside Clinical Trials Unit (TCTU).
The AIR-NET trial is managed by Tayside Clinical Trials Unit (TCTU).
For further information on the trial, including essential trial documentation and training resources, please visit:
