ABOUT US
Welcome to EMBARC
The European Multi-centre Bronchiectasis Audit and Research Collaboration

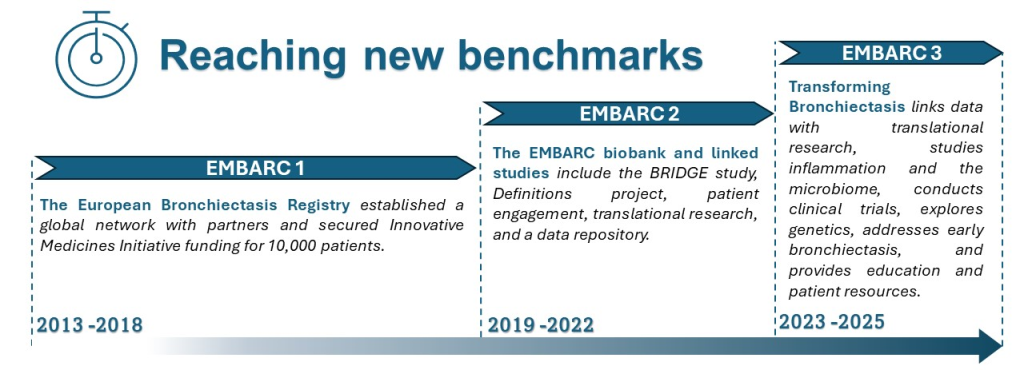
Our History
EMBARC is an international clinical research network committed to promoting clinical research and education in bronchiectasis, through the sharing of protocols, research ideas and expertise.
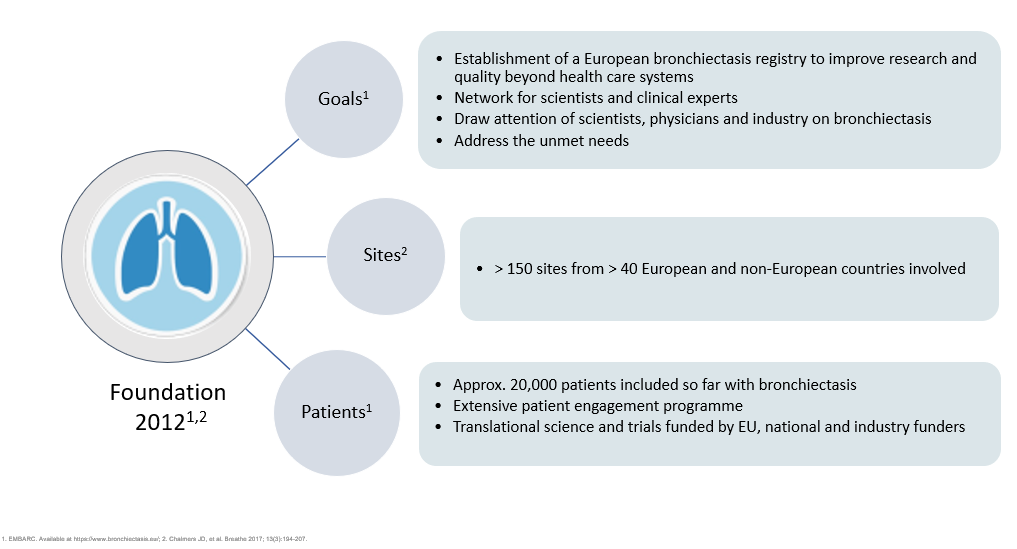
EMBARC was established in 2012 to facilitate multidisciplinary collaborative research in bronchiectasis as a network for scientists and clinical experts to address the unmet needs of patients with bronchiectasis across Europe. Central to this project was the establishment of the European bronchiectasis registry. The EMBARC Registry is open to all investigators around Europe caring for patients with bronchiectasis.
For more than a decade, EMBARC investigators have worked tirelessly to expand and highlight this robust European bronchiectasis registry which now serves as a critical resource for research and quality improvement initiatives.
EMBARC’s aim of raising the awareness of this once neglected disease to the wider attention of physicians, scientists and industry has led to the improvement of patient care and treatment outcomes across diverse healthcare systems
Objectives of the EMBARC network
EMBARC continues to lead the charge in bronchiectasis research, fostering innovation, collaboration, and excellence in clinical practice and scientific discovery through:
- Building on a Distinguished Network: We have cultivated a dynamic network of researchers and clinical experts dedicated to bronchiectasis, providing leadership in guiding research and setting clinical priorities for the future
- Attracting New Talent: EMBARC successfully attracts numerous new researchers and clinicians to the field, fostering a vibrant and growing community of professionals dedicated to advancing bronchiectasis care. If you would like to join the EMBARC registry please contact us
- Supporting Early Career Researchers: We actively support and encourage early career researchers through participation in network activities, promoting their development and integration into the field of bronchiectasis research
- Securing Funding: Our efforts have facilitated successful applications for industry and European Union funding, significantly bolstering bronchiectasis research capacity across Europe.
EMBARC remains a network committed to advancing both clinical and translational science in bronchiectasis bringing together all the countries in Europe but also working closely with our partners across the world.
Why do we need EMBARC?
Bronchiectasis has historically been an under-researched and under-resourced “orphan disease”. It is now recognised that bronchiectasis is placing an increasing burden on healthcare systems around the world and there is an urgent need for better treatments, better clinical care and for clinical and translational research in this condition.
EMBARC was the first truly international bronchiectasis network seeking to bring together investigators from around the world. Central to the project was the creation of a multicentre registry of patients with bronchiectasis. This study brought together a group of experienced researchers across Europe to answer fundamental questions in the epidemiology, aetiology, microbiology, pathophysiology, clinical management and prognosis of bronchiectasis. To date we have recruited and collected data from over 20,000 patients in 31 countries and we are incredibly grateful to all of our investigators who have contributed patient data to the registry.
2023/24 has been the most successful year to date for EMBARC!
In 2023/24, EMBARC:
- published more than 20 high impact publications in 1 year
- presented more than 40 abstracts at leading respiratory conferences including ERS, ATS & WBC
- completed recruitment in two clinical trials testing novel therapeutics in bronchiectasis
- developed additional multi-centre clinical trials which are currently in set up

