ABOUT US
Welcome to EMBARC
EMBARC is a pan-European network committed to promoting clinical research and education in bronchiectasis, through sharing of protocols, research idea and expertise. Central to this project is the creation of the European Bronchiectasis Registry, a collaboration open to all investigators around Europe caring for patients with bronchiectasis.
Our History
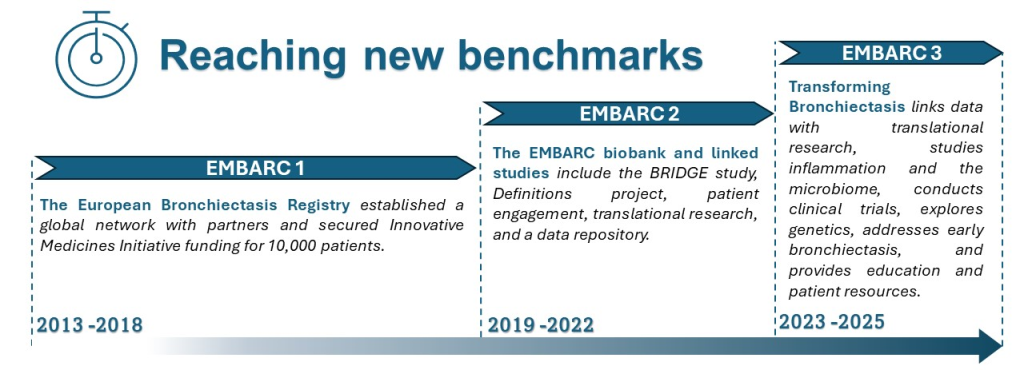
The European Multicentre Bronchiectasis Audit and Research Collaboration network (EMBARC) was established in 2012 to facilitate multidisciplinary collaborative research in bronchiectasis.
Over the past decade, EMBARC has successfully realised its foundational goals:
- Established the EMBARC Registry: We have created and maintained a robust European bronchiectasis registry, serving as a critical resource for research and quality improvement initiatives, thereby enhancing patient care and treatment outcomes across diverse healthcare systems.

- Built a Distinguished Network: We have cultivated a dynamic network of researchers and clinical experts dedicated to bronchiectasis, providing leadership in guiding research and setting clinical priorities for the future
- Attracted New Talent: EMBARC successfully attracts numerous new researchers and clinicians to the field, fostering a vibrant and growing community of professionals dedicated to advancing bronchiectasis care
- Supported Early Career Researchers: We actively support and encourage early career researchers through participation in network activities, promoting their development and integration into the field of bronchiectasis research
- Secured Funding: Our efforts have facilitated successful applications for industry and European Union funding, significantly bolstering bronchiectasis research capacity across Europe
EMBARC continues to lead the charge in bronchiectasis research, fostering innovation, collaboration, and excellence in clinical practice and scientific discovery.
2023/2024 has been the most successful year to date for EMBARC with:
- more than 20 high impact publications in 1 year
- more than 40 abstracts accepted at ERS, ATS & WBC
- completed recruitment in two clinical trials
- further multi-centre clinical trials currently in set-up
Why do we need EMBARC?
Bronchiectasis has historically been an under-researched and under-resourced “orphan disease”. It is now recognised that bronchiectasis is placing an increasing burden on healthcare systems around the world and there is an urgent need for better treatments, better clinical care and for clinical and translational research in this condition.
EMBARC was the first truly international bronchiectasis network seeking to bring together investigators from around the world. Central to the project was the creation of a multicentre registry of patients with bronchiectasis. This study brought together a group of experienced researchers across Europe to answer fundamental questions in the epidemiology, aetiology, microbiology, pathophysiology, clinical management and prognosis of bronchiectasis. To date we have recruited and collected data from over 20,000 patients in 31 countries and we are incredibly grateful to all of our investigators who have contributed patient data to the registry.
The EMBARC Registry
Introduction
When EMBARC was launched in 2012, bronchiectasis was an orphan disease, with few evidence based treatments and a lack of data regarding epidemiology, co-morbidities, pathophysiology, severity and prognosis. There were few longitudinal or cross-sectional studies in bronchiectasis. To give truly meaningful and generalizable results, a longitudinal observational study of bronchiectasis was required to enrol several thousand patients, more than any one centre could enrol. EMBARC has created an open, pan-European registry of patients with bronchiectasis and to date we have collected data from over 20,000 patients in 31 countries making EMBARC the largest registry of bronchiectasis data in the world.

In 2018, we expanded the Registry to include a biobank to gather clinical data and biosamples including blood, DNA, sputum, nasal swabs and urine in order to stimulate translational research and more than 50 projects have been initiated so far using these biosamples.
In recent years we have also started to use the Registry to deliver clinical trials and, in collaboration with one of our industry partners, we have recently completed enrolment of the first EMBARC delivered clinical trial in the UK and Spain looking at a monoclonal antibody against Pseudomonas Aeruginosa.
EMBARC remains a network committed to advancing both clinical and translational science in bronchiectasis bringing together all the countries in Europe but also working closely with our partners across the world. delivering
A collaborative, pan-European database would have several important capabilities, including but not limited to:
- To analyse and assess differences in non_CF bronchiectasis practice across europe and identify areas for improvement or further study
- To analyse factors needing large longitudinal datasets e.g predictors of survival, which cannot be accurately studied from single centre studies
- To allow analyses that cannot be achieved from single centre studies alone, such as identifying the prognosis and features associated with less common forms of bronchiectasis e.g inflammatory bowel disease associated, connective tissue disease, PCD etc.
- Perhaps most importantly, to foster multicentre collaboration in bronchiectasis across Europe and therefore potentially expand the groups activities in time to include recruitment into clinical trials, translational and mechanistic studies
Objectives of the EMBARC network
- To develop a pan-European multicentre bronchiectasis database incorporating baseline data collection with annual follow-up data
- To describe the dempgraphics, co-morbidities, aetiology, medication usage, resource consumption, microbiology, severity and prognosis of bronchiectasis across Europe. In summary, a comprehensive description of characteristics and burden of this disease across the continent
- To foster a collaborative pan-European network that will drive new research and interest in bronchiectasis
Study Design
Participation is open to anyone caring for patients with bronchiectasis. The study is now open with a short term target to enrol over 10,000 patients over the course of 5 years. Baseline data will be recorded using a baseline data form incorporating all relevant bronchiectasis variables. Study participants will then be asked to enter follow up data for patients on an annual basis to give longitudinal data on changes in medication, exacerbation frequency, hospital admissions and survival data.
The data set will be sufficiently simple that any specialised bronchiectasis clinic will be able to provide the data. Nevertheless, the data are sufficiently robust that they will provide a comprehensive overview of all aspects of bronchiectasis care in Europe. The network is intended to serve as a platform to engage researchers and facilitate collaborations around Europe.

Join the EMBARC network now to view the case report form and find further information about taking part in the network activities.
